User:Mr. Ibrahem/Vigabatrin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sabril, Vigadrone, Kigabeq, others |
| Other names | γ-Vinyl-GABA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610016 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Anticonvulsant[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80–90% |
| Protein binding | 0% |
| Metabolism | not metabolized |
| Elimination half-life | 5–8 hours in young adults, 12–13 hours in the elderly. |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
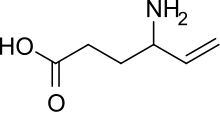
| Formula | C6H11NO2 |
| Molar mass | 129.159 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 171 to 177 °C (340 to 351 °F) |
| |
| |
| (verify) | |
Vigabatrin, sold under the brand name Sabril, is a medication used to treat epilepsy.[1] Specifically it is used for complex partial seizures that are uncontrolled with other measures or for infantile spasms.[1] It is taken by mouth.[1]
Common side effects include headache, tiredness, abdominal pain, vision problems, and swelling.[1] Permanent vision problems occur in about a third of people; with an onset of a month to years after starting treatment.[2][1] It is believed to work by decreasing the breakdown of γ-aminobutyric acid (GABA).[1]
Vigabatrin was approved for medical use in the United States in 2009.[1] It became available as a generic medication in 2019.[3] In the United Kingdom a hundred tablets of 500 mg costs the NHS about £45 as of 2021.[2] In the United States this amount costs about 10,600 USD.[4] In Canada this amount costs about 96 CAD.[5]
References[edit]
- ^ a b c d e f g h i j k "Vigabatrin Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 4 August 2021.
- ^ a b BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 349. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Press Announcements - FDA approves first generic version of Sabril to help treat seizures in adults and pediatric patients with epilepsy". www.fda.gov. Archived from the original on 27 January 2019. Retrieved 21 January 2019.
- ^ "Vigabatrin Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 15 September 2021.
- ^ "Formulary Search - DIN/PIN/NPN Detail". www.formulary.health.gov.on.ca. Archived from the original on 22 September 2021. Retrieved 15 September 2021.
