User:Mr. Ibrahem/Glycopyrronium
 | |
| Clinical data | |
|---|---|
| Trade names | Robinul, Cuvposa, Seebri, others |
| Other names | Glycopyrrolate, glycopyrronium bromide, glycopyrronium tosylate |
| AHFS/Drugs.com | Systemic: Monograph Topical: Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, inhaled, topical |
| Drug class | Antimuscarinic[1] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
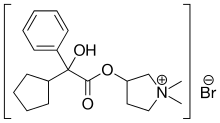
| Formula | C19H28BrNO3 |
| Molar mass | 398.341 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Glycopyrronium, also known as glycopyrrolate, is a medication used to treat COPD, excessive saliva, and excessive sweating.[1][2] It may be taken by mouth, by injection, applied to the skin, or inhaled.[1][2] Effects may last for up to 12 hours.[1] It is also available in combination with a long-acting beta-adrenoceptor agonist (LABA) and inhaled steroid.[2]
Common side effects include dry mouth, urinary retention, blurry vision, large pupils, headache, confusion, sleepiness, and constipation.[1] Other side effects may include allergic reactions and bronchospasm.[1] Safety in pregnancy is unclear.[1] It is an antimuscarinic.[1] It does not generally cross the blood–brain barrier.[1]
Glycopyrronium was approved for medical use in the United States in 1961.[1] It is on the World Health Organization's List of Essential Medicines as an alternative to tiotropium.[3] In the United Kingdom a month of inhaled medication costs the NHS about £28 as of 2021.[2] In the United States the tablets are inexpensive.[4]
References[edit]
- ^ a b c d e f g h i j k l "Glycopyrrolate Monograph for Professionals". Drugs.com. Archived from the original on 16 July 2021. Retrieved 3 December 2021.
- ^ a b c d BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 262. ISBN 978-0857114105.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Glycopyrrolate Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 9 December 2021.
