Functionality (chemistry)
| Monofunctional compounds |
|---|
 |
 |
| Difunctional compounds |
|---|
 |
 |
 |
| Trifunctional compounds |
|---|
 |
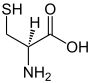 |
In chemistry, functionality is the presence of functional groups in a molecule. A monofunctional molecule possesses one functional group, a bifunctional (or difunctional) two, a trifunctional three, and so forth. In organic chemistry (and other fields of chemistry), a molecule's functionality has a decisive influence on its reactivity.
In polymer chemistry, the functionality of a monomer refers to its number of polymerizable groups, and affects the formation and the degree of crosslinking of polymers.
In organic chemistry and material science[edit]
In organic chemistry, functionality is often used as a synonym for functional group. For example, a hydroxyl group can also be called a HO-function.[1][2]
Functionalisation means the introduction of functional groups, for example
- the functionalisation of a surface[3] (e.g. silanization for the specific modification of the adhesion of a surface)
- the functionalization of nanoparticles of a metal or metal oxide to stabilize such nanoparticles[4] or
- the so-called C-H functionalization,[5] which means the substitution of a C-H bond by a functional group, bonded at the same carbon atom
In polymer chemistry[edit]
According to IUPAC, the functionality of a monomer is defined as the number of bonds that a monomer's repeating unit forms in a polymer with other monomers. Thus in the case of a functionality of f = 2 a linear polymer is formed by polymerizing (a thermoplastic). Monomers with a functionality f ≥ 3 lead to a branching point, which can lead to cross-linked polymers (a thermosetting polymer). Monofunctional monomers do not exist as such molecules lead to a chain termination.[6]
From the average functionality of the used monomers the reaching of the gel point can be calculated as a function of reaction progress.[7] Side reactions may increase or decrease the functionality.[8]
However, IUPAC definition and the use of the term in organic chemistry differ with respect to the functionality of a double bond.[6][9] In polymer chemistry, a double bond possesses a functionality of two (because two points of contact for further polymer chains are present, on each of the two adjacent carbon atoms), while in organic chemistry the double bond is a functional group and thus has a functionality of one.
See also[edit]
References[edit]
- ^ Kurt Peter C. Vollhardt, Neil Eric Schore: Organische Chemie, S. 73 ([1], p. 74, at Google Books).
- ^ Riedel: Moderne Anorganische Chemie von Christoph Janiak, S. 401 ([2], p. 401, at Google Books).
- ^ Alexander Langner, Anthony Panarello, Sandrine Rivillon, Oleksiy Vassylyev, Johannes G. Khinast, Yves J. Chabal: Controlled Silicon Surface Functionalization by Alkene Hydrosilylation, J. Am. Chem.
- ^ Marie-Alexandra Neouze, Ulrich Schubert: Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands, Monatsh.
- ^ Dirk Steinborn: Grundlagen der metallorganischen Komplexkatalyse, S. 305 ([3], p. 239, at Google Books
- ^ a b Eintrag zu functionality, f of a monomer.
- ^ Koltzenburg: Polymere: Synthese, Eigenschaften und Anwendungen, S. 187 ([4], p. 188, at Google Books). This reference is being translated to English as "Polymer Chemistry" by the same authors, to appear in September 2017. See [5]
- ^ Hans-Georg Elias: Makromoleküle: Chemische Struktur und Synthesen, S. 468 und 477 ([6], p. 468, at Google Books).
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "chemical functionality". doi:10.1351/goldbook.CT07503
