User:Mr. Ibrahem/Octreotide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sandostatin, Bynfezia Pen, Mycapssa, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693049 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous, intramuscular, intravenous, by mouth |
| Drug class | Somatostatin[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60% (IM), 100% (SC) |
| Protein binding | 40–65% |
| Metabolism | Liver |
| Elimination half-life | 1.7–1.9 hours |
| Excretion | Urine (32%) |
| Identifiers | |
| |
| Chemical and physical data | |
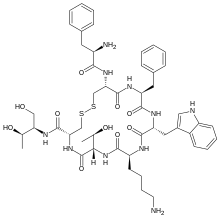
| Formula | C49H66N10O10S2 |
| Molar mass | 1019.25 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Octreotide, sold under the brand name Sandostatin among others, is a medication used to treat carcinoid syndrome, acromegaly, congenital hyperinsulinism, and gastrointestinal bleeding due to esophageal varices.[1][2] It can be given by injection into a vein or muscle.[1] The immediate release form may be effective up to 12 hours.[1]
Common side effects include nausea, a slow heart rate, and abdominal discomfort.[1] Other side effects may include low or high blood sugar, gall bladder problems, and anaphylaxis.[1] It is unclear if use during pregnancy is safe.[3] Use is not recommended when breastfeeding.[4] It mimics the effects of natural somatostatin and inhibits the release of growth hormone, serotonin, insulin, and glucagon.[1]
It was first made in 1979, by the chemist Wilfried Bauer.[5] It was approved for medical use in the United States in 1988.[6] It is available as a generic medication.[4] In the United Kingdom it costs about 3 pounds per 50 ug dose as of 2020.[4] In the United States this amount costs about 5.30 USD as of 2020.[7]
References[edit]
- ^ a b c d e f g h i "Octreotide Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 25 December 2020.
- ^ Cat, TB; Liu-DeRyke, X (September 2010). "Medical management of variceal hemorrhage". Critical care nursing clinics of North America. 22 (3): 381–93. doi:10.1016/j.ccell.2010.02.004. PMID 20691388.
- ^ "Octreotide Use During Pregnancy". Drugs.com. Archived from the original on 20 January 2021. Retrieved 25 December 2020.
- ^ a b c BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1002. ISBN 978-0-85711-369-6.
- ^ MD, Laura Lamps; MD, Andrew Bellizzi; MD, Wendy L. Frankel; MD, Rhonda Yantiss; MD, Scott R. Owens (2015). Neoplastic Gastrointestinal Pathology: An Illustrated Guide. Springer Publishing Company. p. 40. ISBN 978-1-936287-72-7. Archived from the original on 28 August 2021. Retrieved 25 December 2020.
- ^ "Octreotide Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 25 December 2020.
- ^ "Octreotide Prices and Octreotide Coupons". GoodRx. Retrieved 25 December 2020.
