User:Ricech/sandbox
Bibliography Links: Need 5 references by next tuesday[edit]
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2195025/pdf/737.pdf
http://www.jbc.org/content/236/2/405.full.pdf
https://www.ncbi.nlm.nih.gov/pubmed/15491403
http://pubs.acs.org/doi/pdf/10.1021/bi00577a012
http://pubs.acs.org/doi/abs/10.1021/bi401126z
co binds 250 times more tightly than o2
- Hall, John E. (2010). Guyton and Hall textbook of medical physiology (12th ed.). Philadelphia, Pa.: Saunders/Elsevier. p. 502. ISBN 978-1416045748.
- Perutz, Max (1998). "1–11". Science is Not a Quiet Life. World Scientific. ISBN 9789814498517.
See also pages[edit]
Oxygen–haemoglobin dissociation curve
other[edit]
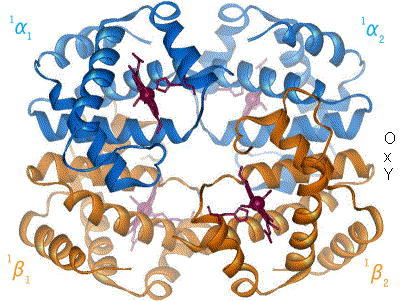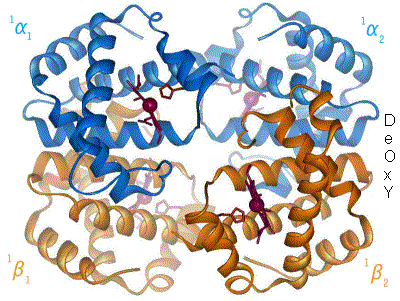
The oxygen-haemoglobin dissociation curve shifts to the right when the carbon dioxide or hydrogen ion concentration is increased, signifying a decrease in haemoglobin oxygen affinity.


Outline[edit]
Hi, I'm a student with Wiki Ed, working to improve biochemistry-linked articles. I've come up with some changes I'd like to make over the next week or two, and I thought I'd post a basic outline for what I'm hoping to do. If anyone has any questions, concerns, or feedback, feel free to let me know here or on my talk page. Thanks!
Outline[edit]
Introduction:
-Largely would stay the same, possibly some minor edits to improve continuity with changes in the overall structure of the article.
History and Experimental Discovery of the Bohr Effect:
-In his paper, Bohr cites several previous experiments, conducted by both him and his contemporaries, which consist of failed models and important building blocks for the eventual determination of the effect. I'll organize these successive experiments to get a timeline of the discoveries leading up to the discovery of the Bohr effect.
- There is some controversy over whether Bronislav Verigo independently discovered the effect in 1898, six years prior to Bohr. I haven't yet found any definitive proof of this, but the controversy itself should be noted.
-Include a summary of Bohr's experimental procedures and how he conclusively proved the relationship between carbon dioxide, pH, and haemoglobin oxygen affinity.
Cooperativity between Haemoglobin Subunits and Allosteric Regulation:
-Expand on current allostery section, with explanation of cooperativity in haemoglobin and its physiological effects (only cooperative effects, Bohr effect gets its own section) and elaboration on T and R states.
Mechanism:
-A few changes to current mechanism section, mostly added emphasis on how H+ and CO2 interact with and stabilize the T-state (i.e. identify specific key residues that form salt bridges)
Physiological Role:
-This section overlaps quite a bit with the mechanism section, so I'll trim off the more mechanistic bits and add them to the other section, while focusing exclusively on physiological effects in this one.
-Evidence shows that the magnitude of the Bohr effect is inversely related to the body size of an animal, so I'd discuss this here
Special Cases:
-Carbon monoxide, which is a competitive inhibitor (occupies oxygen binding sites on haemoglobin)
-Marine mammals (diving ability may be linked to modifications to standard Bohr effect)
Bohr Effect[edit]
From Wikipedia, the free encyclopedia
Not to be confused with the Bohr Equation.
Haemoglobin Dissociation Curve. Dotted red line corresponds with shift to the right caused by Bohr effect
The Bohr effect is a physiological phenomenon first described in 1904 by the Danish physiologist Christian Bohr, stating that haemoglobin's oxygen binding affinity (see Oxygen–haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide.[1] That is, an increase in blood CO2 concentration, which leads to a decrease in blood pH, will result in haemoglobin proteins releasing their load of oxygen. Conversely, a decrease in carbon dioxide provokes an increase in pH, which results in haemoglobin picking up more oxygen.
Experimental Discovery[edit]
In the early 1900s, Christian Bohr was a professor at the University of Copenhagen in Denmark, already well known for his work in the field of respiratory physiology.[2] He had spent the last two decades studying the solubility of oxygen, carbon dioxide, and other gases in various liquids,[3] and had conducted extensive research on haemoglobin and its affinity for oxygen.[2] In 1903, he began working closely with Karl Hasselbalch and August Krogh, two of his associates at the university, in an attempt to experimentally replicate the work of Gustav von Hüfner, using whole blood instead of haemoglobin solution.[1] Hüfner had suggested that the oxygen-haemoglobin binding curve was hyperbolic in shape,[4] but after extensive experimentation, the Copenhagen group determined that the curve was in fact sigmoidal. Furthermore, in the process of plotting out numerous dissociation curves, it soon became apparent that high partial pressures of carbon dioxide caused the curves to shift to the right.[3] Further experimentation while varying the CO2 concentration quickly provided conclusive evidence, confirming the existence of what would soon become known as the Bohr effect.[1]
Controversy[edit]
There is some debate over whether Bohr was actually the first to discover the relationship between CO2 and oxygen affinity, or whether the Russian physiologist Bronislav Verigo beat him to it, allegedly discovering the effect in 1898, six yeas before Bohr.[5] While this has never been proven, Verigo did in fact publish a paper on the haemoglobin-CO2 relationship in 1892.[6] However, his proposed model was flawed, and Bohr harshly criticized it in his own publications.[1]
Another challenge to Bohr's discovery comes from within his lab. Though Bohr was quick to take full credit, his associate Krogh, who invented the apparatus used to measure gas concentrations in the experiments,[7] maintained throughout his life that he himself had actually been the first to demonstrate the effect. Though there is some evidence to support this, retroactively changing the name of a well-known phenomenon would be extremely impractical, so it remains known as the Bohr effect.[3]
Physiological role[edit]
The Bohr effect increases the efficiency of oxygen transportation through the blood. After haemoglobin binds to oxygen in the lungs due to the high oxygen concentrations, the Bohr effect facilitates its release in the tissues, particularly those tissues in most need of oxygen. When a tissue's metabolic rate increases, so does its carbon dioxide waste production. When released into the bloodstream, carbon dioxide forms bicarbonate through the following reaction:
Although this reaction usually proceeds very slowly, the enzyme carbonic anhydrase (which is present in red blood cells) drastically speeds up the conversion to bicarbonate and protons.[8] This causes the pH of the blood to decrease, which promotes the dissociation of oxygen from haemoglobin, and allows the surrounding tissues to obtain enough oxygen to meet their demands. Conversely, in the lungs, where oxygen concentration is high, binding of oxygen causes haemoglobin to release protons, which recombine with bicarbonate to release carbon dioxide during exhalation. Since these two reactions directly oppose one another, they balance each other out, and there is little overall change in blood pH.
The Bohr effect enables the body to adapt to changing conditions and makes it possible to supply extra oxygen to tissues that need it the most. For example, when muscles are undergoing strenuous activity, they require large amounts of oxygen to conduct cellular respiration, which generates CO2 (and therefore HCO3− and H+) as byproducts. These waste products lower the pH of the blood, which increases oxygen delivery to the active muscles. Carbon dioxide is not the only molecule that can trigger the Bohr effect. If muscle cells aren't receiving enough oxygen for cellular respiration, they resort to lactic acid fermentation, which releases lactic acid as a byproduct. This increases the acidity of the blood even more than CO2 alone, which reflects the cells' even greater need for oxygen. In fact, under anaerobic conditions, muscles generate lactic acid so quickly that pH of the blood passing through the muscles will drop to around 7.2, which causes haemoglobin to begin releasing ~10% more oxygen.[8]
Relationship with Body Size[edit]
The magnitude of the Bohr effect is usually given by , and exhibits an inverse relationship with the size of an organism: the magnitude increases as size and weight decreases. For example, mice possess a very strong Bohr effect, with a of 0.96, which requires relatively minor changes in H+ or CO2 concentrations, while elephants require much larger changes in concentration to achieve a much weaker effect = 0.38.[9]
Mechanism[edit]
Allosteric Interactions[edit]
The Bohr effect hinges around allosteric interactions between the hemes of the haemoglobin tetramer, a mechanism first proposed by Max Perutz in 1970.[10] Haemoglobin exists in two conformations: a high-affinity R state and a low-affinity T state. When oxygen concentration levels are high, as in the lungs, the R state is favored, enabling the maximum amount of oxygen to be bound to the hemes. In the capillaries, where oxygen concentration levels are lower, the T state is favored, in order to facilitate the delivery of oxygen to the tissues. The Bohr effect is dependent on this allostery, as increases in CO2 and H+ help stabilize the T state and ensure greater oxygen delivery to muscles during periods of elevated cellular respiration. This is evidenced by the fact that myoglobin, a monomer with no allostery, does not exhibit the Bohr effect.[8] Haemoglobin mutants with weaker allostery may exhibit a reduced Bohr effect. For example, in Hiroshima variant haemoglobinopathy, allostery in haemoglobin is reduced, and the Bohr effect is diminished. As a result, during periods of exercise, the mutant haemoglobin has a higher affinity for oxygen and tissue may suffer minor oxygen starvation.[11]
T State Stabilization[edit]
When haemoglobin is in its T state, the N-terminal amino groups of the α-subunits and the C-terminal histidine of the β-subunits are protonated, giving them a positive charge and allowing these residues to participate in ionic interactions with carboxyl groups on nearby residues. These ion pairs stabilize the charges on the residues, and help hold the haemoglobin in the T state. Decreases in pH stabilize this state even more, since they make these residues even less likely to be deprotonated, which would cause them to lose their charges, and thus break up the ionic interactions. In the R state, the ionic pairings are absent, meaning that the R state's stability increases when the pH increases, as it makes these residues more likely to stay deprotonated. The Bohr effect works by simultaneously destabilizing the high-affinity R state and stabilizing the low-affinity T state, which leads to a overall decrease in oxygen affinity.[8] This can be visualized on an oxygen-haemoglobin dissociation curve by shifting the whole curve to the right.
Carbon dioxide can also react directly with the N-terminal amino groups to form carbamates, according to the following reaction:
CO2 forms carbamates more frequently with the T state, which helps to stabilize this conformation. The process also creates protons, meaning that the formation of carbamates also contributes to the strengthening of ionic interactions, further stabilizing the T state.[8]
Special Cases[edit]
Marine Mammals[edit]
An exception to the otherwise well-supported link between animal body size and the sensitivity of its haemoglobin to changes in pH was discovered in 1961[12] Based on their size and weight, many marine mammals were hypothesized to have a very low, almost negligible Bohr effect.[9] However, when their blood was examined, this was not the case. Humpback whales weighing 41,000 kilograms had an observed of 0.82, which is roughly equivalent to the Bohr effect magnitude in a 0.57 kg guinea pig.[9] This extremely strong Bohr effect is hypothesized to be one of marine mammals' many adaptations for deep, long dives, as it allows for virtually all of the bound oxygen on haemoglobin to dissociate and supply the whale's body while it is underwater.[12] Examination of other marine mammal species supports this. In pilot whales and porpoises, which are primarily surface feeders and seldom dive for more than a few minutes, the was 0.52, comparable to a cow,[9] which is much closer to the expected Bohr effect magnitude for animals of their size.[12]
Carbon Monoxide[edit]
Another special case of the Bohr effect occurs when carbon monoxide is present. This molecule serves as a competitive inhibitor for oxygen, and binds to haemoglobin to form carboxyhaemoglobin.[13] haemoglobin's affinity for CO is about 250 times stronger than its affinity for O2,[14] meaning that is very unlikely to dissociate, and once bound, it blocks the binding of O2 to that subunit. At the same time, CO is structurally similar enough to O2 to cause carboxyhaemoglobin to favor the R state, raising the oxygen affinity of the remaining unoccupied subunits. This combination significantly reduces the delivery of oxygen to the tissues of the body, which is what makes carbon monoxide so toxic. Interestingly, though, this toxicity is reduced slightly by an increase in the strength of the Bohr effect in the presence of carboxyhaemoglobin. This increase is ultimately due to differences in interactions between heme groups in carboxyhaemoglobin relative to oxygenated haemoglobin. It is most pronounced when the oxygen concentration is extremely low, as a last-ditch effort when the need for oxygen delivery becomes critical. The physiological implications of this phenomenon are remain unclear.[13]
References[edit]
- ^ a b c d Bohr; Hasselbalch, Krogh. "Concerning a Biologically Important Relationship - The Influence of the Carbon Dioxide Content of Blood on its Oxygen Binding".
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Irzhak, L. I. "Christian Bohr (On the Occasion of the 150th Anniversary of His Birth)". Human Physiology. 31 (3): 366–368. doi:10.1007/s10747-005-0060-x. ISSN 0362-1197.
- ^ a b c "Blood and Hemoglobin: The Evolution of Knowledge of Functional Adaptation in a Biochemical System. Part I: The Adaptation of Chemical Structure to Function in Hemoglobin on JSTOR" (PDF). www.jstor.org. Retrieved 2016-11-08.
- ^ G. Hüfner, "Ueber das Gesetz der Dissociation des Oxyharmoglobins und iiber einige daran sich knupfenden wichtigen Fragen aus der Biologie," Arch. Anat. Physiol. (Physiol. Abtheilung) (1890), 1-27.
- ^ "Вериго эффект - это... Что такое Вериго эффект?". Словари и энциклопедии на Академике. Retrieved 2016-11-08.
- ^ B. Werigo, "Zur Frage uber die Wirkung des Sauerstoffs auf die Kohlensaureausscheidung in den Lungen," Pflugers Arch. ges. Physiol., 51 (1892), 321-361.
- ^ A. Krogh, "Apparat und Methoden zur Bestimmung der Aufnahme von Gasen im Blute bei verschiedenen Spannungen der Gase," Skand. Arch. Physiol., 16 (1904), 390-401.
- ^ a b c d e Voet, Donald; Judith G. Voet; Charlotte W. Pratt (2013). Fundamentals of Biochemistry: Life at the Molecular Level (4th ed.). John Wiley & Sons, Inc. p. 189.
- ^ a b c d Riggs, Austen (1960-03-01). "The Nature and Significance of the Bohr Effect in Mammalian Hemoglobins". The Journal of General Physiology. 43 (4): 737–752. doi:10.1085/jgp.43.4.737. ISSN 0022-1295. PMID 19873527.
- ^ Perutz, Max. Science is Not a Quiet Life. World Scientific. ISBN 9789814498517.
- ^ Olson, JS; Gibson QH; Nagel RL; Hamilton HB (December 1972). "The ligand-binding properties of hemoglobin Hiroshima ( 2 2 146asp )". The Journal of Biological Chemistry. 247 (23): 7485–93. PMID 4636319.
- ^ a b c Riggs, Austen (1961-04-01). "Bohr Effect in the Hæmoglobins of Marine Mammals". Nature. 190 (4770): 94–95. doi:10.1038/190094a0.
- ^ a b Hlastala, M. P.; McKenna, H. P.; Franada, R. L.; Detter, J. C. (1976-12-01). "Influence of carbon monoxide on hemoglobin-oxygen binding". Journal of Applied Physiology. 41 (6): 893–899. ISSN 0021-8987. PMID 12132.
- ^ Hall, John E. (2010). Guyton and Hall Textbook of Medical Physiology (12th ed.). Philadelphia, Pa: Saunders/Elsevier. p. 502. ISBN 978-1416045748.
{{cite book}}: no-break space character in|title=at position 47 (help)





