User:Mr. Ibrahem/Tazemetostat
 | |
| Clinical data | |
|---|---|
| Trade names | Tazverik, others |
| Other names | EPZ-6438 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620018 |
| License data |
|
| Routes of administration | By mouth[1] |
| Drug class | EZH2 inhibitor[1] |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Chemical and physical data | |
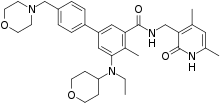
| Formula | C34H44N4O4 |
| Molar mass | 572.750 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tazemetostat, sold under the brand name Tazverik, is a medication used to treat epithelioid sarcoma which cannot be removed by surgery and non-Hodgkin lymphoma that has failed other treatments.[1] It is taken by mouth.[1]
Common side effects include pain, tiredness, nausea, decreased appetite, vomiting, and constipation.[1] Other side effects may include secondary cancers including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.[2] It works by blocking the activity of EZH2 .[3]
Tazemetostat was approved for medical use in the United States in 2020.[1] As of 2021 has not been fully approved in Europe; though has been granted an orphan designation.[4][5] In the United States it costs about 17,300 USD per month as of 2021.[6]
References[edit]
- ^ a b c d e f g h "Tazemetostat Monograph for Professionals". Drugs.com. Archived from the original on 11 July 2021. Retrieved 24 September 2021.
- ^ "FDA approves first treatment option specifically for patients with epithelioid sarcoma, a rare soft tissue cancer". U.S. Food and Drug Administration (FDA) (Press release). 23 January 2020. Archived from the original on 25 December 2020. Retrieved 23 January 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Lue JK, Amengual JE (October 2018). "Emerging EZH2 Inhibitors and Their Application in Lymphoma". Curr Hematol Malig Rep. 13 (5): 369–382. doi:10.1007/s11899-018-0466-6. PMID 30112706. S2CID 52010283.
- ^ "Tazemetostat". SPS - Specialist Pharmacy Service. 17 September 2019. Archived from the original on 24 May 2021. Retrieved 24 September 2021.
- ^ "EU/3/18/2004: Orphan designation for the treatment of diffuse large B-cell lymphoma". Archived from the original on 24 September 2021. Retrieved 24 September 2021.
- ^ "Tazverik Prices, Coupons and Patient Assistance Programs". Retrieved 24 September 2021.
