User:Mr. Ibrahem/Rucaparib
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ruːˈkæpərɪb/ roo-KAP-ər-ib |
| Trade names | Rubraca |
| Other names | AG014699 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617002 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| Drug class | PARP inhibitor[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30–45% (Tmax = 1.9 hours) |
| Protein binding | 70% (in vitro) |
| Metabolism | Liver (primarily CYP2D6; 1A2 and 3A4 to a lesser extent) |
| Elimination half-life | 17–19 hours[1] |
| Identifiers | |
| |
| Chemical and physical data | |
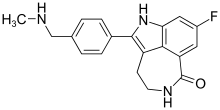
| Formula | C19H18FN3O |
| Molar mass | 323.371 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rucaparib, sold under the brand name Rubraca, is a medication used for ovarian cancer, fallopian tube cancer, peritoneal cancer, and prostate cancer.[1][3] It is used after other treatments have failed.[4] It is taken by mouth.[1]
Common side effects include tiredness, nausea, kidney problems, liver problems, low red blood cells, abnormal taste, diarrhea, low platelets, and abdominal pain.[4] Other side effects may include acute myeloid leukemia and low neutrophils.[5] Use during pregnancy may harm the baby.[5] It is a PARP inhibitor, which block DNA repair in cells with mutation in the BRCA gene.[1]
Rucaparib was approved for medical use in the United States in 2016 and Europe in 2018.[5][4] In the United States it costs about 9,100 USD per month as of 2021.[6]
References[edit]
- ^ a b c d e f "DailyMed - RUBRACA- rucaparib tablet, film coated". dailymed.nlm.nih.gov. Archived from the original on 9 May 2020. Retrieved 19 October 2021.
- ^ "Rubraca 200mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 19 June 2019. Archived from the original on 29 August 2021. Retrieved 17 May 2020.
- ^ a b "Rucaparib Camsylate - National Cancer Institute". www.cancer.gov. 3 January 2017. Archived from the original on 9 July 2021. Retrieved 19 October 2021.
- ^ a b c d e "Rubraca". Archived from the original on 6 August 2020. Retrieved 19 October 2021.
- ^ a b c "Rucaparib Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 19 October 2021.
- ^ "Rubraca Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 March 2021. Retrieved 19 October 2021.
