User:Mr. Ibrahem/Imipramine
 | |
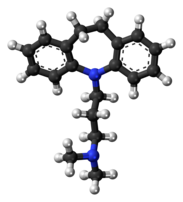 | |
| Clinical data | |
|---|---|
| Trade names | Tofranil, Tofranil-PM, others |
| Other names | Imipramine hydrochloride, melipramine; G-22355 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682389 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular injection |
| Drug class | Tricyclic antidepressant (TCA)[2] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 94–96%[3] |
| Protein binding | 86%[4] |
| Metabolism | Liver (CYP1A2, CYP2C19, CYP2D6)[4] |
| Metabolites | Desipramine[4] |
| Elimination half-life | 20 hours[4] |
| Excretion | Kidney (80%), fecal (20%)[4] |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C19H24N2 |
| Molar mass | 280.415 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Imipramine, sold under the brand name Tofranil, among others, is a tricyclic antidepressant (TCA) used to treat depression, anxiety disorders including panic disorder, bed wetting, and postherpetic neuralgia.[5][2] It is taken by mouth.[2]
Common side effects include dry mouth, sleepiness, constipation, blurry vision, and low blood pressure with standing.[2] Other side effects may include mania, sunburns, seizures, and suicide.[2] Safety in pregnancy is unclear.[6] How it works is not clear but may involve increasing levels of serotonin and norepinephrine.[2]
Imipramine was discovered in 1951 and came into medical use in 1959.[7] It was the first TCA to be marketed.[8] It is available as a generic medication. In the United Kingdom 4 weeks of 25 mg costs the NHS about £7 as of 2021.[5] This amount in the United States is about 10 USD.[9]
References[edit]
- ^ a b "Imipramine Use During Pregnancy". Drugs.com. 28 August 2019. Archived from the original on 7 February 2020. Retrieved 7 February 2020.
- ^ a b c d e f g h "Imipramine Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 26 November 2021.
- ^ Heck HA, Buttrill SE Jr, Flynn NW, Dyer RL, Anbar M, Cairns T, Dighe S, Cabana BE (June 1979). "Bioavailability of imipramine tablets relative to a stable isotope-labelled internal standard: increasing the power of bioavailability tests". Journal of Pharmacokinetics and Biopharmaceutics. 7 (3): 233–248. doi:10.1007/bf01060015. PMID 480146. S2CID 23232584.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e "Product Information Tolerade (imipramine hydrochloride)". TGA eBusiness Services. PMIP Pty Ltd. 4 June 2013. Archived from the original on 10 December 2019. Retrieved 16 October 2013.
- ^ a b c BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 397. ISBN 978-0857114105.
- ^ "Imipramine Use During Pregnancy". Drugs.com. Archived from the original on 7 February 2020. Retrieved 26 November 2021.
- ^ Walker, S. R. (6 December 2012). Trends and Changes in Drug Research and Development. Springer Science & Business Media. p. 109. ISBN 978-94-009-2659-2. Archived from the original on 14 September 2016. Retrieved 26 November 2021.
- ^ "imipramine | Uses, Mechanism of Action, & Side Effects | Britannica". www.britannica.com. Archived from the original on 8 November 2021. Retrieved 26 November 2021.
- ^ "Imipramine Hydrochloride Prices and Imipramine Hydrochloride Coupons - GoodRx". GoodRx. Retrieved 26 November 2021.
