User:Mr. Ibrahem/Fondaparinux
 | |
| Clinical data | |
|---|---|
| Trade names | Arixtra |
| Other names | Fondaparinux sodium |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Subcutaneous |
| Drug class | Factor Xa inhibitor[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 94% |
| Metabolism | renally excreted unchanged |
| Elimination half-life | 17-21 hours[2] |
| Chemical and physical data | |
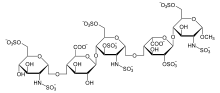
| Formula | C31H43N3Na10O49S8 |
| Molar mass | 1728.03 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fondaparinux, sold under the brand name Arixtra, is an anticoagulant used to treat and prevent blood clots (deep vein, pulmonary, and superficial vein thrombosis) and to treat unstable angina and heart attacks.[1][3] It is given by injection under the skin.[3]
Common side effects include bleeding.[1] Other side effects may include low platelets.[1] Use is not recommended in those with serious kidney problems.[3] There is no evidence of harm to the baby with use in pregnancy.[1] It works by blocking factor Xa.[3]
Fondaparinux was approved for medical use in the United States in 2001 and Europe in 2002.[1][3] It is available as a generic medication.[4] In the United Kingdom 2.5 mg costs the NHS about £6.[4] In the United States this amount costs about 20 USD.[5]
References[edit]
- ^ a b c d e f g h "Fondaparinux Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 13 December 2021.
- ^ Walenga JM, Jeske WP, Fareed J (2005). "Biochemical and Pharmacologic Rationale for Synthetic Heparin Polysaccharides". Chemistry and Biology of Heparin and Heparan Sulfate. Elsevier. pp. 143–177. doi:10.1016/b978-008044859-6/50006-x. ISBN 978-0-08-044859-6.
The elimination half-life of AT-bound fondaparinux is 17–21 h (171,172). The subcutaneous bioavailability of fondaparinux is nearly 100% and it is distributed mainly in the blood (165,173).
- ^ a b c d e f "Arixtra". Archived from the original on 21 June 2021. Retrieved 13 December 2021.
- ^ a b BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 139. ISBN 978-0857114105.
- ^ "Fondaparinux Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 10 April 2021. Retrieved 13 December 2021.
