User:Mr. Ibrahem/Fluoxymesterone
 | |
| Clinical data | |
|---|---|
| Trade names | Androxy, Halotestin, Ora-Testryl, Ultandren, others |
| Other names | Fluoxymestrone; Androfluorene; NSC-12165 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682690 |
| Pregnancy category |
|
| Routes of administration | By mouth[1] |
| Drug class | Androgen; Anabolic steroid |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | By mouth: 80%[2] |
| Metabolism | Liver (6β-hydroxylation, 5α- and 5β-reduction, 3α- and 3β-keto-oxidation, 11β-hydroxy-oxidation)[3] |
| Metabolites | • 5α-Dihydrofluoxymesterone[3] • 11-Oxofluoxymesterone[3] |
| Elimination half-life | 9.2 hours[4][5] |
| Excretion | Urine (<5% unchanged)[2][3] |
| Identifiers | |
| |
| Chemical and physical data | |
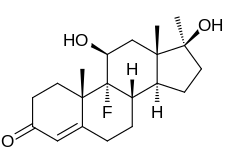
| Formula | C20H29FO3 |
| Molar mass | 336.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fluoxymesterone, sold under the brand names Androxy among others, is a medication used to treat low testosterone in men, delayed puberty in boys, and breast cancer in women.[6] It is taken by mouth.[6]
Common side effects in females include acne, increased hair growth, voice changes, and increased aggressiveness while common side effects in males include breast enlargement and increased erections.[6][1] Other side effects may include liver damage, heart failure, high blood calcium, and priapism.[6] Use during pregnancy may harm the baby.[6] It is a manufactured androgen and anabolic steroid.[6]
Fluoxymesterone was approved for medical use in the United States in 1956.[6] In the United States 100 tablets of 10 mg costs about 415 USD as of 2021.[7] In has also been used to improve physique and performance.[6] The medication is a controlled substance in many countries such that non-medical use is generally illicit.[1]
References[edit]
- ^ a b c d e William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 500–508. ISBN 978-0-9828280-1-4. Archived from the original on 2021-02-15. Retrieved 2021-09-17.
- ^ a b Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1360–. ISBN 978-1-60913-345-0.
- ^ a b c d Kammerer RC, Merdink JL, Jagels M, Catlin DH, Hui KK (1990). "Testing for fluoxymesterone (Halotestin) administration to man: identification of urinary metabolites by gas chromatography-mass spectrometry". J. Steroid Biochem. 36 (6): 659–66. doi:10.1016/0022-4731(90)90185-u. PMID 2214783.
- ^ Seth Roberts (2009). Anabolic Pharmacology.
- ^ Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1279–. ISBN 978-0-7817-6879-5. Archived from the original on 2017-09-08. Retrieved 2021-09-17.
- ^ a b c d e f g h i j k "Fluoxymesterone Monograph for Professionals". Drugs.com. Archived from the original on 20 November 2021. Retrieved 14 December 2021.
- ^ "Androxy Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 14 December 2021.
