User:Mr. Ibrahem/Fenofibrate
 | |
| Clinical data | |
|---|---|
| Trade names | Fenoglide, Lipofen, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601052 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99% |
| Metabolism | glucuronidation |
| Elimination half-life | 20 h |
| Excretion | urine (60%), feces (25%) |
| Identifiers | |
| |
| Chemical and physical data | |
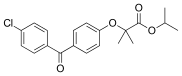
| Formula | C20H21ClO4 |
| Molar mass | 360.83 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 80 to 81 °C (176 to 178 °F) |
| |
| |
| (verify) | |
Fenofibrate, sold under the brand name Tricor among others, is a medication of the fibrate class used to treat abnormal blood lipid levels.[3] It is less preferred to statin medications as it does not appear to reduce the risk of heart disease or death.[3][4] Its use is recommended together with dietary changes.[3] It is taken by mouth.[3]
Common side effects include liver problems, breathing problems, abdominal pain, muscle problems, and nausea.[3] Serious side effects may include toxic epidermal necrolysis, rhabdomyolysis, gallstones, blood clots, and pancreatitis.[3] Use in pregnancy and breastfeeding is not recommended.[4][5] It works by a number of mechanisms.[3]
It was patented in 1969, and came into medical use in 1975.[6] It is available as a generic medication.[4] A month supply in the United Kingdom costs the NHS about 3.67 £ per month as of 2019.[4] In the United States the wholesale cost of this amount is about US$8.40.[7] In 2018, it was the 73rd most commonly prescribed medication in the United States with more than 11 million prescriptions.[8][9]
References[edit]
- ^ "Fenofibrate 267mg Capsules - Summary of Product Characteristics (SmPC)". (emc). 12 February 2020. Retrieved 13 April 2020.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Retrieved 7 September 2020.
- ^ a b c d e f g "Fenofibric Acid/Fenofibrate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ a b c d British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 198. ISBN 9780857113382.
- ^ "Fenofibrate Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 474. ISBN 9783527607495.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- ^ "The Top 300 of 2021". ClinCalc. Retrieved 18 February 2021.
- ^ "Fenofibrate - Drug Usage Statistics". ClinCalc. Retrieved 18 February 2021.
