User:Kjorgen4/sandbox
 | This is a user sandbox of Kjorgen4. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Tight junctions, also known as occluding junctions or zonulae occludentes (singular, zonula occludens) are multiprotein junctional complexes whose general function is to prevent leakage of transported solutes and water and seals the paracellular pathway. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. Tight junctions are present only in vertebrates. The corresponding junctions that occur in invertebrates are septate junctions.
Structure[edit][edit]
Tight junctions are composed of a branching network of sealing strands, each strand acting independently from the others. Therefore, the efficiency of the junction in preventing ion passage increases exponentially with the number of strands. Each strand is formed from a row of transmembrane proteins embedded in both plasma membranes, with extracellular domains joining one another directly. There are at least 40 different proteins composing tight junctions. These proteins consist of both transmembrane and cytoplasmic proteins. The three major transmembrane proteins are occludin, claudins, and junction adhesion molecule (JAM) proteins. These associate with different peripheral membrane cytoplasmic proteins such as ZO-1 located on the intracellular side of plasma membrane, which anchor the strands to the actin component of the cytoskeleton. Thus, tight junctions join together the cytoskeletons of adjacent cells.

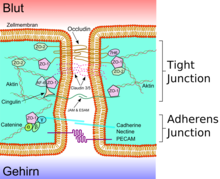
Transmembrane proteins:
- Occludin was the first integral membrane protein to be identified. It has a molecular weight of ~60kDa. It consists of four transmembrane domains and both the N-terminus and the C-terminus of the protein are intracellular. It forms two extracellular loops and one intracellular loop. It plays a key role in cellular structure and barrier function.[1]

- Claudins were discovered after occludin and are a family of 24 different proteins. They have a molecular weight of ~20kDa. They have a structure similar to that of occludin in that they have four transmembrane domains and similar loop structure. They are understood to be the backbone of tight junctions and play a significant role in the tight junction's ability to seal. Different claudins are found in different locations throughout the human body.
- Junction Adhesion Molecules (JAM) are part of the immunoglobulin superfamily. They have a molecular weight of ~40kDa. Their structure differs from that of the other integral membrane proteins in that they only have one transmembrane domain instead of four.
Functions[edit][edit]
They perform vital functions:
- They hold cells together.
- Barrier function, which can be further subdivided into protective barriers and functional barriers serving purposes such as material transport and maintenance of osmotic balance:
- Tight junctions help to maintain the polarity of cells by preventing the lateral diffusion of integral membrane proteins between the apical and lateral/basal surfaces, allowing the specialized functions of each surface (for example receptor-mediated endocytosis at the apical surface and exocytosis at the basolateral surface) to be preserved. This aims to preserve the transcellular transport.
- Tight junctions prevent the passage of molecules and ions through the space between plasma membranes of adjacent cells, so materials must actually enter the cells (by diffusion or active transport) in order to pass through the tissue. Investigation using freeze-fracture methods in electron microscopy is ideal for revealing the lateral extent of tight junctions in cell membranes and has been useful in showing how tight junctions are formed. The constrained intracellular pathway exacted by the tight junction barrier system allows precise control over which substances can pass through a particular tissue. (Tight junctions play this role in maintaining the blood–brain barrier.) At the present time, it is still unclear whether the control is active or passive and how these pathways are formed. In one study for paracellular transport across the tight junction in kidney proximal tubule, a dual pathway model is proposed: large slit breaks formed by infrequent discontinuities in the TJ complex and numerous small circular pores.
In human physiology there are two main types of epithelia using distinct types of barrier mechanism. Epidermal structures such as skin form a barrier from many layers of keratinized squamous cells. Internal epithelia on the other hand more often rely on tight junctions for their barrier function. This kind of barrier is mostly formed by only one or two layers of cells. It was long unclear whether tight cell junctions also play any role in the barrier function of the skin and similar external epithelia but recent research suggests that this is indeed the case.
Classification[edit][edit]
Epithelia are classed as "tight" or "leaky", depending on the ability of the tight junctions to prevent water and solute movement:
- Tight epithelia have tight junctions that prevent most movement between cells. Examples of tight epithelia include the distal convoluted tubule, the collecting duct of the nephron in the kidney, and the bile ducts ramifying through liver tissue.
- Leaky epithelia do not have these tight junctions, or have less complex tight junctions. For instance, the tight junction in the kidney proximal tubule, a very leaky epithelium, has only two to three junctional strands, and these strands exhibit infrequent large slit breaks.
References for article:
**The tight junction: a multifunctional complex https://www.physiology.org/doi/full/10.1152/ajpcell.00558.2003
-24 members of the mammalian claudin family.
**Molecular Physiology and Pathophysiology of Tight Junctions I. Tight junction structure and function: lessons from mutant animals and proteins https://www.physiology.org/doi/full/10.1152/ajpgi.2000.279.2.G250
-Tight junctions are made up in large part by the proteins occludin and claudin. There are now 24 different mammalian claudins that have been identified all belonging to the claudin family. Claudins and occludin play a vital role in the the TJs ability to seal the paracellular space. There is evidence that claudins play the largest role in establishing the paracellular barrier.
**Physiology and Function of the Tight Junction http://cshperspectives.cshlp.org/content/1/2/a002584.full.pdf+html
-TJs have been found to contain over 40 different types of proteins.
Molecular structure and assembly of the tight junction https://www.physiology.org/doi/abs/10.1152/ajprenal.1998.274.1.f1
**Tight Junction in Blood-Brain Barrier: An Overview of Structure, Regulation, and Regulator Substances http://web.b.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=0&sid=be4b693c-4f90-41bc-bc20-b5e24b629f07%40sessionmgr104
-TJs make up basic structure of the BBB in regards to limiting paracellular permeability. Good explanation of both occludin and claudins.
**Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation https://www.ncbi.nlm.nih.gov/pubmed/23140302
-Claudin 3 and 5 important for BBB integrity. JAMs involved in cell polarity.
**Tight junctions of the blood–brain barrier: development, composition and regulation https://www.sciencedirect.com/science/article/pii/S1537189102002008
-Good references to discovery of different TJ components.
- ^ Liu, Wei-Ye; Wang, Zhi-Bin; Zhang, Li-Chao; Wei, Xin; Li, Ling (2012-06-12). "Tight Junction in Blood-Brain Barrier: An Overview of Structure, Regulation, and Regulator Substances". CNS Neuroscience & Therapeutics. 18 (8): 609–615. doi:10.1111/j.1755-5949.2012.00340.x. ISSN 1755-5930.
