User:Ezpzii/Reductive amination
| This is the sandbox page where you will draft your initial Wikipedia contribution.
If you're starting a new article, you can develop it here until it's ready to go live. If you're working on improvements to an existing article, copy only one section at a time of the article to this sandbox to work on, and be sure to use an edit summary linking to the article you copied from. Do not copy over the entire article. You can find additional instructions here. Remember to save your work regularly using the "Publish page" button. (It just means 'save'; it will still be in the sandbox.) You can add bold formatting to your additions to differentiate them from existing content. |
Article Draft[edit]
Reductive amination (also known as reductive alkylation) is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is usually a ketone or an aldehyde. It is a common method to make amines and is widely used in green chemistry since it can be done catalytically in one-pot under mild conditions. In biochemistry, dehydrogenase enzymes use reductive amination to produce the amino acid, glutamate. Additionally, there is ongoing research on alternative synthesis mechanisms which various metal catalysts which require more mild reaction conditions, allowing the reaction to be less energy taxing. Investigation into biocatalysts, such as lRED, have allowed for the reduction of chiral amines which is an important factor in pharmaceutical synthesis.[1]
Reaction process[edit]
Reductive amination occurs between a carbonyl such as an aldehyde or ketone and an amine in the presence of a reducing agent.[2] The reaction conditions are neutral or weakly acidic.[2]

The amine first reacts with the carbonyl group to form a hemiaminal species which subsequently loses one molecule of water in a reversible manner by alkylimino-de-oxo-bisubstitution to form the imine intermediate.[3] The equilibrium between aldehyde/ketone and imine is shifted toward imine formation by dehydration.[2] This intermediate imine can then be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride) to produce the final amine product.[2] Intramolecular reductive amination can also occur to afford a cyclic amine product if the amine and the carbonyl are on the same molecule of the starting material.[2]
There are two ways to conduct a reductive amination reaction: direct and indirect.[2]
Direct Reductive Amination[edit]
In a direct reaction, the carbonyl and amine starting materials and the reducing agent are combined and the reductions are done sequentially.[2] These are often one pot reactions since the imine intermediate is not isolated before the final reduction to the product.[2] Instead, as the reaction proceeds, the imine becomes favoured for reduction over the carbonyl starting material.[2] The two most common methods for direct reductive amination are hydrogenation with catalytic platinum, palladium, or nickel catalysts, and the use of hydride reducing agents like cyanoborohydride (NaBH3CN).[2]
Indirect Reductive Amination[edit]
Indirect reductive amination, also called a stepwise reduction, isolates the imine intermediate.[2] In a separate step, the isolated imine intermediate is reduced to form the amine product.[2]
Designing a reductive amination reaction[edit]
There are many considerations to be made when designing a reductive amination reaction.[4]
- Chemoselectivity issues may arise since the carbonyl group is also reducible.
- The reaction between the carbonyl and amine are in equilibrium, with favouring for the carbonyl side unless water is removed from the system.
- Reducible intermediates may appear in the reaction which can affect chemoselectivity.
- The amine substrate, imine intermediate or amine product might deactivate the catalyst.
- Acyclic imines have E/Z isomers. This makes it difficult to create enantiopure chiral compounds through stereoselective reductions.
To solve the last issue, asymmetric reductive amination reactions can be used to synthesize an enantiopure product of chiral amines.[4] In asymmetric reductive amination, a carbonyl that can be converted from achiral to chiral is used.[5] The carbonyl undergoes condensation with an amine in the presence of H2 and a chiral catalyst to form the imine intermediate which is then reduced to form the amine.[5] However, this method is still limiting to synthesize primary amines which are non-selective and prone to overalkylation.[5]
Common reducing agents[edit]
Sodium Borohydride[edit]
NaBH4 reduces both imines and carbonyl groups.[3] However, it is not very selective and can reduce other reducible functional groups present in the reaction.[3] To ensure that this does not occur, reagents with weak electrophilic carbonyl groups, poor nucleophilic amines and sterically hindered reactive centres should not be used, as these properties do not favour the reduction of the carbonyl to form an imine and increases the chance that other functional groups will be reduced instead.[3]
Sodium Cyanoborohydride[edit]
Sodium cyanoborohydride is soluble in hydroxylic solvents, stable in acidic solutions, and has different selectivities depending on the pH.[2] At low pH values, it efficiently reduces aldehydes and ketones.[6] As the pH increases, the reduction rate slows and instead, the imine intermediate becomes preferential for reduction.[6] For this reason, NaBH3CN is an ideal reducing agent for one-pot direct reductive amination reactions that don't isolate the intermediate imine.[2]
When used as a reducing agent, NaBH3CN can release toxic by-products like HCN and NaCN during work up.[2]
H2/metal Catalyst[edit]
H2/Pt and H2/Pd are the two most common hydrogen/metal catalysts, but other metals such as Raney Nickel, Cobalt, Copper and Iron are also used.[7] H2/metal catalysts are a greener method compared to sodium cyanoborohydride since borate by-products are not produced and the metal catalysts can be recovered and reused.[7]
In green chemistry[edit]
Reductive amination is commonly used over other methods such as SN2 type reactions with halides to produce an alkylamines since it can be done in mild conditions and has high selectivity for nitrogen-containing compounds.[8][9] Reductive amination can occur sequentially in one-pot reactions, which eliminates the need for intermediate purifications and reduces waste.[8] Some multistep synthetic pathways have been reduced to one step through one-pot reductive amination.[8] This makes it a highly appealing method to produce amines in green chemistry.
[edit]
Additionally, there exists many systems which catalyze reductive amination with a hydrogenation catalyst.[10] Generally, catalysis is preferred to stoichiometric reactions to enable the reaction to be more efficient, more atom economic, and to produce less waste.[11] This can be either a homogeneous catalytic system or heterogeneous system.[10] These systems provide an alternative method which is efficient, requires fewer volatile reagents and is redox economic.[10][12] As well, this method can be used in the reduction of alcohols, along with aldehydes and ketones to form the amine product.[10] One example of a heterogeneous catalytic system is the reductive amination of alcohols using the Ni-catalyzed system.[10]

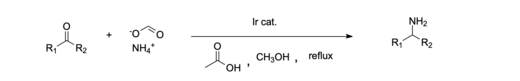
Nickel is commonly used as a catalyst for reductive amination because of its abundance and relatively good catalytic activity.[10][13] An example of a homogeneous catalytic system is the reductive amination of ketones done with an iridium catalyst.[14] Additionally, it has been shown to be effective to use a homogeneous Iridium (III) catalyst system to reductively aminate carboxylic acids, which in the past has been more difficult than aldehydes and ketones.[11] Homogeneous catalysts are often favored because they are more environmentally and economically friendly compared to most heterogeneous systems. [10]
Biochemistry[edit]
In biochemistry, dehydrogenase enzymes can catalyze the reductive amination of α-keto acids and ammonia to yield α-amino acids. Reductive amination is predominantly used for the synthesis of the amino acid glutamate starting from α-ketoglutarate, while biochemistry largely relies on transamination to introduce nitrogen in the other amino acids. The use of enzymes as a catalyst is advantageous because the enzyme active sites are often stereospecific and have the ability to selectively synthesize a certain enantiomer.[15] This is useful in the pharmaceutical industry, particularly for drug-development, because enantiomer pairs can have different reactivities in the body.[1][16] Additionally, enzyme biocatalysts are often quite selective in reactivity so they can be used in the presence of other functional groups, without the use of protecting groups.[17][15] For instance a class of enzymes called imine reductase, IRED, can be used to catalyze direct asymmetric reductive amination to form chiral amines.[1][17]
References[edit]
- ^ a b c Thorpe, Thomas W.; Marshall, James R.; Harawa, Vanessa; Ruscoe, Rebecca E.; Cuetos, Anibal; Finnigan, James D.; Angelastro, Antonio; Heath, Rachel S.; Parmeggiani, Fabio; Charnock, Simon J.; Howard, Roger M.; Kumar, Rajesh; Daniels, David S. B.; Grogan, Gideon; Turner, Nicholas J. (6 April 2022). "Multifunctional biocatalyst for conjugate reduction and reductive amination". Nature. 604 (7904): 86–91. doi:10.1038/s41586-022-04458-x. ISSN 1476-4687.
- ^ a b c d e f g h i j k l m n o Abdel-Magid, Ahmed F.; Carson, Kenneth G.; Harris, Bruce D.; Maryanoff, Cynthia A.; Shah, Rekha D. (1996-01-01). "Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures 1". The Journal of Organic Chemistry. 61 (11): 3849–3862. doi:10.1021/jo960057x. ISSN 0022-3263.
- ^ a b c d Tripathi, Rama P.; Verma, Shyam S.; Pandey, Jyoti; Tiwari, Vinod K. "Recent Development on Catalytic Reductive Amination and Applications". Current Organic Chemistry. 12 (13): 1093–1115. doi:10.2174/138527208785740283.
- ^ a b Wang, Chao; Xiao, Jianliang (2013), Li, Wei; Zhang, Xumu (eds.), "Asymmetric Reductive Amination", Stereoselective Formation of Amines, vol. 343, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 261–282, doi:10.1007/128_2013_484. pmid: 24158548., ISBN 978-3-642-53928-2, retrieved 2023-11-06
{{citation}}: Check|doi=value (help) - ^ a b c Reshi, Noor U Din; Saptal, Vitthal B.; Beller, Matthias; Bera, Jitendra K. (2021-11-19). "Recent Progress in Transition-Metal-Catalyzed Asymmetric Reductive Amination". ACS Catalysis. 11 (22): 13809–13837. doi:10.1021/acscatal.1c04208. ISSN 2155-5435.
- ^ a b Borch, Richard F.; Durst, H. Dupont (1969-07). "Lithium cyanohydridoborate, a versatile new reagent". Journal of the American Chemical Society. 91 (14): 3996–3997. doi:10.1021/ja01042a078. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ a b "Hydrogen/metal catalysts (precious and base metal)". reagents.acsgcipr.org. Retrieved 2023-12-06.
- ^ a b c Van Praet, Sofie; Preegel, Gert; Rammal, Fatima; Sels, Bert F. (2022-05-12). "One-Pot Consecutive Reductive Amination Synthesis of Pharmaceuticals: From Biobased Glycolaldehyde to Hydroxychloroquine". ACS Sustainable Chemistry & Engineering. 10 (20): 6503–6508. doi:10.1021/acssuschemeng.2c00570. ISSN 2168-0485.
- ^ He, Jian; Chen, Lulu; Liu, Shima; Song, Ke; Yang, Song; Riisager, Anders (2020). "Sustainable access to renewable N-containing chemicals from reductive amination of biomass-derived platform compounds". Green Chemistry. 22 (20): 6714–6747. doi:10.1039/d0gc01869d. ISSN 1463-9262.
- ^ a b c d e f g Huang, Hao; Wei, Yuejun; Cheng, Yuran; Xiao, Shuwen; Chen, Mingchih; Wei, Zuojun (7 October 2023). "The Acquisition of Primary Amines from Alcohols through Reductive Amination over Heterogeneous Catalysts". Catalysts. 13 (10): 1350. doi:10.3390/catal13101350. ISSN 2073-4344.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Ouyang, Lu; Miao, Rui; Yang, Zhanhui; Luo, Renshi (2023-02-01). "Iridium-catalyzed reductive amination of carboxylic acids". Journal of Catalysis. 418: 283–289. doi:10.1016/j.jcat.2023.01.030. ISSN 0021-9517.
- ^ Burns, Noah Z.; Baran, Phil S.; Hoffmann, Reinhard W. (2009-04-06). "Redox Economy in Organic Synthesis". Angewandte Chemie International Edition. 48 (16): 2854–2867. doi:10.1002/anie.200806086. ISSN 1433-7851.
- ^ Chernyshev, Victor M.; Ananikov, Valentine P. (2022-01-21). "Nickel and Palladium Catalysis: Stronger Demand than Ever". ACS Catalysis. 12 (2): 1180–1200. doi:10.1021/acscatal.1c04705. ISSN 2155-5435.
- ^ Tanaka, Kouichi; Miki, Takashi; Murata, Kunihiko; Yamaguchi, Ayumi; Kayaki, Yoshihito; Kuwata, Shigeki; Ikariya, Takao; Watanabe, Masahito (2019-09-06). "Reductive Amination of Ketonic Compounds Catalyzed by Cp*Ir(III) Complexes Bearing a Picolinamidato Ligand". The Journal of Organic Chemistry. 84 (17): 10962–10977. doi:10.1021/acs.joc.9b01565. ISSN 0022-3263.
- ^ a b Wohlgemuth, Roland; Littlechild, Jennifer (22 July 2022). "Complexity reduction and opportunities in the design, integration and intensification of biocatalytic processes for metabolite synthesis". Frontiers in Bioengineering and Biotechnology. 10. doi:10.3389/fbioe.2022.958606. ISSN 2296-4185. PMC 9355135. PMID 35935499.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Brooks, W. H.; Guida, W. C.; Daniel, K. G. "The Significance of Chirality in Drug Design and Development". Current Topics in Medicinal Chemistry. 11 (7): 760–770. doi:10.2174/156802611795165098. PMC 5765859. PMID 21291399.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Wu, Kai; Huang, Junhai; Shao, Lei (2022-11-22). "Imine Reductases: Multifunctional Biocatalysts with Varying Active Sites and Catalytic Mechanisms". ChemCatChem. 14 (22). doi:10.1002/cctc.202200921. ISSN 1867-3880.
