Talk:Supercapacitor
| This is the talk page for discussing improvements to the Supercapacitor article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| This It is of interest to the following WikiProjects: | |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||
| The contents of the Electric double-layer capacitor page were merged into Supercapacitor. For the contribution history and old versions of the redirected page, please see its history; for the discussion at that location, see its talk page. (2018-05-30) |
|
This article links to one or more target anchors that no longer exist.
Please help fix the broken anchors. You can remove this template after fixing the problems. | Reporting errors |
ambiguous[edit]
The section where it says;
With regards to rechargeable batteries supercapacitors feature higher peak currents, low cost per cycle, no danger of overcharging, good reversibility, non-corrosive electrolyte and low material toxicity, while batteries offer, lower purchase cost, stable voltage under discharge, but they require complex electronic control and switching equipment, with consequent energy loss and spark hazard given a short.
Which technology requires the complex electronic control? — Preceding unsigned comment added by 198.103.184.76 (talk) 21:03, 29 March 2019 (UTC)
- For what it's worth, I agree that the statement in that section is still ambiguous, and no knowledgeable person has responded to your observation yet, which is surprising given the quality of the article itself. I'd guess that the supercapacitor requires the complex electronic control and switching equipment (because of their higher energy density?), but that's just an uninformed guess, so someone who knows should hopefully respond. UnderEducatedGeezer (talk) 06:23, 22 April 2019 (UTC)
Change in understanding, from 'Electric double-layer capacitor' to 'Supercapacitor'[edit]
Electric double-layer capacitors (EDLCs), invented 1957, have seen a dramatic change in understanding of their capacitive charge storage from a pure physical function between Helmholtz double-layers to an additional pseudocapacitive chemical charge storage with redox reactions, electrosorption and intercalation processes. Because nowadays (2013) an electrochemical capacitor is not only an EDLC anymore the question comes up how to name this very special capacitors.
Generally in science publications all the different developments of the last years are united under the term “electrochemical capacitors”. But if a development gets a discrete component from a manufacturer, the names are manifold. Supercap, Ultracap, Goldcap, Greencap, a lot of manufacturer related names exist.
A look through the science literature of electrochemical capacitors shows, that roughly 70 to 80 % of the authors uses the term “Supercapacitor”. (see: A Bibliometric Analysis of the International Literature in Supercapacitors, Francesco Lufrano* and Pietro Staiti, Int. J. Electrochem. Sci., 4 (2009) 173 – 186 PDF)
A google research gives 730,000 results for Supercapacitor, for Ultracapacitor only 363,000 but for Double-layer capacitor Google gives 1,540,000 results. (Date: 2013-05-07)

That means, that as of 2013 the term double-layer capacitor is used in public linguistic usage, the term supercapacitor in science publications. Because in the broad public knowledge the new development of pseudocapacitance is not known, otherwise the science results are clear and available, that every EDLC not only has a double-layer capacitance but also a pseudocapacitance and nearly all new developments are trying to enhance the properties of the capacitors by increasing the pseudocapacitance the term double-layer capacitor is not correct anymore.
This is the reason this article now use the term supercapacitor. The reasons why in the science the term supercapacitor is prevailed instead of the term ultracapacitor are surely first the respect for B. E. Conway, who coined the term supercapacitor, and the second reason may be that the often used term “ultracapacitor” is used by Maxwell, the market leader, like a trade name for their capacitors, and that smells a little bit like advertising.
Please help to better this detailed and bulky article, written by a German, by editing grammar, style, cohesion, tone, or spelling.--Elcap (talk) 07:29, 16 May 2013 (UTC)
Please fix this sentence:[edit]
The article presently contains the following sentence which is not easy to understand, partly because it needs punctuation. I can't edit it because I'm not sure what it is supposed to mean: "All this first electrochemical capacitors used a cell design of two aluminum foils covered with activated carbon coins the electrodes which are soaked with an electrolyte and separated by a thin porous insulator implemented in a common housing." Edison (talk) 19:23, 21 May 2013 (UTC)
--Elcap (talk) 17:50, 30 May 2013 (UTC)
Copyedit +[edit]
Copyediting this. Feedback encouraged. Comments:
- This is WAY too long. 5k words would be more like it, rather than the current 17k. The way forward is to split this into multiple articles, with summaries in this one. I'll list split candidates as I go.
- Create a History of supercapacitors article with this article's History section.
- Hi Lfstevens, first thank you for correcting my German colored language. But please be careful with changing technical terms or technical figures. For example, your proposal to create an article of History of superconductance use a wrong term, superconductance is something happen near minus 270 degrees and has nothing to do with the way from double-layer capacitance to pseudocapacitance. In some cases I have seen you change figures. Please notice, that I like to use figures coming from datasheets and not from science publications. Figures from datasheets you can trust on, figures from science publications often are without encapsulation, that are developments not capacitors. I feel free to rechange that, what I believe is right --Elcap (talk) 10:59, 24 May 2013 (UTC)
- Sorry, of course I meant History of supercapacitance. I'm happy to correct any errors I introduce and welcome your feedback. If you prefer to make the corrections or other changes instead, go for it.
- I'm not sure about the phrase "static in origin". Is the point about the origin, or would it be better said as "static in nature" or just "static" or even "electrostatic".
- "Because both capacitances have approximately the same value, the total capacitance is roughly half the capacitance of one electrode." Shouldn't it be double rather than half?
- Hi, I never believe that you ask me that. The two electrodes are connected in serial. The formula for serial connected capacitors is
Please set C1 = 100 µF and C2 = 100 µF and fill in the formula. The result gives the answer --Elcap (talk) 17:50, 30 May 2013 (UTC)
- "the permittivity ε is 6 (instead of 80 in normal conditions)". What are "normal conditions"?
- This: "An essential fundamental difference from redox reactions in batteries arises in supercapacitors, were a fast sequence of reversible redox processes with a linear function of degree of faradaic charge transfers take place." does not make sense. What thing is a linear function of the degree of faradaic charge transfers? Or is it that faradaic charge transfers are a linear function of time? I went with "Compared with batteries, supercapacitor faradaic processes are much faster and leave fewer reaction products with a linear function of degree of faradaic charge transfers."
- Good question. The redox reactions in supercapacitors depend on number of transferred electrons coming from “specifically adsorbed ions”. I will change the paragraph into:
- “An essential fundamental difference from redox reactions in batteries arises in supercapacitors, were a fast sequence of reversible redox processes, electrosorption or intercalation processes with faradaic electron charge transfers take place without any phase changes of the electrode molecules. The ions or atoms simply cling to the atomic structure of the electrode without making or breaking of chemical bonds. This behavior is the basic function of a new class of capacitance, the pseudocapacitance. Pseudocapacitance comprise fast and reversible faradaic processes with charge electron transfer between electrolyte and the electrode leads to a linear capacitive behavior and is accomplished in combination with the nonfaradaic formation of an electric double-layer which both contribute indivisible to the total capacitance of the capacitor. Capacitors with a high amount of pseudocapacitance are called pseudocapacitors. --Elcap (talk) 17:50, 30 May 2013 (UTC)
- And linear with respect to what? And some tweaks:
- A fundamental difference between faradaic reactions in batteries and in supercapacitors is that in the latter, the reactions are reversible redox, electrosorption or intercalation processes without phase changes or making/breaking chemical bonds. The ions or atoms are adsorbed to the electrode. This behavior is the essence of a new class of capacitance, termed "pseudocapacitance". Such processes lead to a linear capacitive behavior and are accomplished in combination with (nonfaradaic) electric double-layer capacitance. Capacitors with a high amount of pseudocapacitance are called pseudocapacitors. --
- “An essential fundamental difference from redox reactions in batteries arises in supercapacitors, were a fast sequence of reversible redox processes, electrosorption or intercalation processes with faradaic electron charge transfers take place without any phase changes of the electrode molecules. The ions or atoms simply cling to the atomic structure of the electrode without making or breaking of chemical bonds. This behavior is the basic function of a new class of capacitance, the pseudocapacitance. Pseudocapacitance comprise fast and reversible faradaic processes with charge electron transfer between electrolyte and the electrode leads to a linear capacitive behavior and is accomplished in combination with the nonfaradaic formation of an electric double-layer which both contribute indivisible to the total capacitance of the capacitor. Capacitors with a high amount of pseudocapacitance are called pseudocapacitors. --Elcap (talk) 17:50, 30 May 2013 (UTC)
- Is "underpotential deposition of hydrogen or metaladatoms in surface lattice sites" meant to be the definition of "electrosorption"? The term needs a definition, and I couldn't find one in IUPAC. Is electrosorption the same as "electro-adsorption"? Couldn't find that one either.
- The Ragone plot shows far less power density for SCs than does [8].
- Yes, I draw this Ragone plot by myself basing on values coming from datasheets, not from science publications. I describe the supercapacitors as an industrial product. New developments have normally 30 % higher energy density values than commercial available products. --Elcap (talk) 17:50, 30 May 2013 (UTC)
- WP defines faradaic as purely redox reactions. In this article and in the above link, intercalation and electrosorption are also included. Which one is right?
- Answer, see ref Conway, Pell, page 643, 644. Please ask the authors of “Faradaic” whether they really describe the last cognitions.--Elcap (talk) 17:50, 30 May 2013 (UTC)
- I looked at the Conway piece. He refers to this def: "The current (or current density) that is flowing through an electrochemical cell and is causing (or is caused by) chemical reactions (charge transfer) occurring at the electrode surfaces." I left a note on the faradaic talk page.
- Answer, see ref Conway, Pell, page 643, 644. Please ask the authors of “Faradaic” whether they really describe the last cognitions.--Elcap (talk) 17:50, 30 May 2013 (UTC)
- " The atoms or ions contribute to the pseudocapacitance simply cling to the atomic structure of the electrode and charges are distributed on surfaces by physical adsorption processes that do not involve the making or breaking of chemical bonds." I interpret this to mean that these redox reactions do not involve the making or breaking of chemical bonds. If so, what is happening? And what is the nature of the clinging? Adsorption? I had enough trouble with the pseudocapacitance section that I'm posting my proposal here to avoid damaging the article.
More questions[edit]
- Split the materials sections into the articles for EDLCs and pseudocapacitors, respectively, with short summaries in the main article.
- That articles separately that powdered and solid activated carbon are the most popular.
- The article uses terms like "high" and very high" without defining them. As such, they are meaningless. Generally, I've removed them. If a specific definition is available, we need to cite it.
- What is a "cascaded RC element"?
- Can't understand "To exploit the entire capacity of all pores up to the end of the electrode, all individual pore capacitances have to be achieved via the several serial RC time constants. These result in a delayed current flow, which makes the time or frequency dependent value of the total capacitance. Or, with other words, thus, the total capacitance of supercapacitors only achieved after longer measuring times."
- For the explanation you have to calculate let me say 4 RC elements in series connection with decreasing capacitance and increasing resistance. For every RC element you get a charge or discharge time (in seconds after 4 time constants), f. e. 10 s, 50 s, 100 s 500 s. So at least after 660 s the capacitor is totally charged or discharged.--Elcap (talk) 17:18, 14 June 2013 (UTC)
- Split the applications section into Supercapacitor applications.
- Add a different comparison table by anode type, cathode type, electrolyte, voltage showing best reported energy density, power density, DL cap, Pseudeo cap, total cap, ESR, (usable?) surface area/m2 and /g, pore size, etc.
- please give me a research time of let me say 50 to 70 years, I repeat “YEARS” and the admission to visit all manufactures and universities round the world and perhaps you will get this table with approximately 15,000 lines, valid only for a few days. Sorry, but this good theoretical question can't be anwered because nobody have acces to the secret developments of the manufacturers.--Elcap (talk) 17:18, 14 June 2013 (UTC)
- "the internal time constant from ESR" needs to be defined.
- "Calculated with this formula capacitors, specified with 5000 h at 65 °C, may have an estimated operation life time of 20,000 h at 45 h." What does the 45h refer to?
- What is a "durty" effect?
- "The self-discharge rate is, for most applications, sufficiently low enough, but it is higher than in accumulators." What is an accumulator?
- in German an “Akkumulator” is a rechargeable battery like in English Accumulator (energy)--Elcap (talk) 17:18, 14 June 2013 (UTC)
- "Low cost per cycle" purchase, operating or both?
- It is a complicated calculation. Because the SCs are much more expensive than batteries but on the other hand they can survive much more charge/discharge cycles and live much longer, the marketing people of the SC manufacturers have calculated the cost per cycle, that include normally purchasing and operating, but operating cost are not clearly to calculate: man power?, energy losses?, service?--Elcap (talk) 17:18, 14 June 2013 (UTC)
- If voltage drops with discharge, does it increase with charge?
- Suggest merging the "new developments" into the main body. "New" gets old quickly.
- The Conway article mentions that Mercury is the best-characterized metal. Might be worth mentioning.
Waiting for feedback... Lfstevens (talk) 09:45, 24 May 2013 (UTC)
Hi Lfstevens, I am back from my holyday now.
One remark to that you you make in your copyedit. Wiki writes to Copyedit: Copy editing (also copy-editing, copyediting) is the work that an editor does to improve the formatting, style, and accuracy of text. Unlike general editing, copy editing might not involve changing the substance of the text.
What you are doing is changing of the text, changing the sense of this article. I do not agree in splitting the article into two or more articles.
This article is long, of course, but it is as long as is necessary to understand the very complex way from the principles of charge storing up to the manyfold different types, their characteristics, their applications, their new developments and their market figures. I hope, you can follow, that this complete new family of capacitors forms an unit only to understand for a reader if all aspects are together in one article.
- I did not split the article, nor would I do so without achieving a consensus. Splitting does make sense. Many far more complex topics (e.g., World War I) have "head" articles that are much shorter than this. Please consider your audience. You're not writing for chemists or device makers, but for general audiences. This is an encyclopedia, not a textbook. Very few individual articles should exceed a few thousand words. Lfstevens (talk) 08:18, 30 May 2013 (UTC)
You have a lot of questions, I try to answer all. But please stop editing for a while until I repair a lot of changes you have made which either are technical not correct or not understood. Regards--Elcap (talk) 07:31, 30 May 2013 (UTC)
- You are welcome to fix my errors (I am not a chemist.) However, I volunteer to fix them myself if you'll explain them to me. I'm done editing until my questions are answered. Lfstevens (talk) 08:18, 30 May 2013 (UTC)
- Hi Lfstevens, thank you so much for all your time you spend to edit this article. You can see, that I have accepted most of your proposals. For some paragraphs like capacitance, energy and power density etc. I saw, that my old text was not clear enough, so you cannot formulate a correct edited version. Please have a look at the new version, I think, now it is better to understand. Regards from sunny Germany --Elcap (talk) 17:18, 14 June 2013 (UTC)
Hi Lfstevens, your make the remark the current article is too long. This article is a translation from the German one, as long as the English, but for the German one nobody had said it is to long. But to follow your remark I am trying to write an own article about Pseudocapacitance (including development of the historical part) analogue to Capacitance and shorten the paragraph in this article. Do you think it is a compromise? --Elcap (talk) 11:03, 26 June 2013 (UTC)
- I missed this, but then I noticed the new copyedit tag. I think that is a good step forward. I am not familiar with German WP, but I have found that English readers are better served by the strategy of giving them just enough info to get them interested and pointing the way so they can drill down for more. This strategy is employed by many articles in many subject areas. If the article is stable, I'm happy to take another copyedit pass. My level of understanding has improved slightly so I expect I will be able to avoid some of my first round mistakes. Lfstevens (talk) 06:31, 4 September 2013 (UTC)
- Hi LfStevens, I am glad to read, that your understanding in capacitors has improved a little bit. To split article in several articles, it is the same in the English Wikis as in the German Wiki. It works for articles like 2. World War, because all battles and historical things easily could described in single articles, everybody can see the coherence. But please, here you have a capacitor in your hand, that is one closed case with two terminals. Do really want to separate articles like "Electrode materials for supercapacitors"?
- I had agreed for an own article "Pseudocapacitance", I never forgot, but give me some time, I am on the way.
- One additional remark with regard to the length of the article. Since more than 40 years now I am working for and with capacitors in the industry. The very special view for my capacitor articles here comparing with other Wiki articles for electronic components is the part or section of describing the electrical parameters, how to measure and so on. These informations are based on international standards (IEC). That are important informations normally nobody knows or easily can find. Especially for supercapacitors some manufacturers have their own, not standardized measure procedures. That is important for customers to know this. And this section is vital for understanding the working of the "one closed case with two terminals". Otherwise you can not separate these section combining all "capacitor parameter measuring procedures" in one separate article, because every capacitor has different standardized procedures how and what to measure. It is my very special industrial view of components lengthens the the article, but I believe, that my view is vital to describe the complete and complex functionality of a component. --Elcap (talk) 07:41, 5 September 2013 (UTC)
Discussion new article "Pseudocapacitance"[edit]

Pseudocapacitance arises from faradaic electrochemical processes rather than the electrostatic mechanism of traditional capacitors. It differs from battery chemistry in that it mostly does not involve making or breaking chemical bonds. Instead it comes from specific redox reactions, electrosorption and intercalation.[1][2] The atoms or ions contributing the pseudocapacitance cling[3] to the atomic structure of the electrode and charges are distributed on surfaces by physical adsorption processes. If the electrode materials consist of transition metal oxides, pseudocapacitance enhances the value of specific capacitance ca. 10 -100 times.[2]
Redox reactions in rechargeable batteries are well characterized. These processes are associated with chemical reactions of the electrode materials usually with attendant phase changes. Although these processes are mostly reversible, repeated charge/discharge cycles accumulate unreversed reaction products, limiting cycle-life. Further, the low speed of the reactions extends charge and discharge times and lowers power density.
Compared with batteries, supercapacitor faradaic processes are much faster and leave fewer reaction products with a linear function of degree of faradaic charge transfers.
Applying a voltage at the capacitor terminals, the ions in the electrolyte move to the opposite electrode to form a double-layer. Pseudocapacitance originates at the anode when specifically adsorbed cations invade the double-layer, producing an excess of electrons in several single-electron stages. The electrons are transferred to or from valence-electron states (orbitals) of the reagent. The electrons enter the anode and flow through the external circuit to the cathode, where a double-layer with an equal number of anions has formed. These anions do accept the electrons. The anions remain on the surface of the electrode in the charged state, and the electrons remain in the ionized and "electron hungry" transition-metal ions of the electrode. Pseudocapacitance has a linear function within narrow limits and is determined by the potential-dependent degree of coverage of surface with the adsorbed anions from the electrolyte. Pseudocapacitance storage capacity is limited by the quantity and available surface of the reagent.
Under discharge the reaction of charge transfer is reversed and the ions or atoms leave the double-layer distributing randomly into the electrolyte.
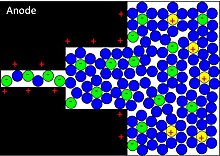
The ability of electrodes to accomplish redox reactions of electroactive species, electrosorption of H or metal ad-atoms or intercalation, strongly depend on the chemical affinity of electrode materials to the ions sorbed on the electrode surface as well as on the structure and dimension of the electrode pores. Transition-metal oxides have the potential for pseudocapacitance. They are inserted by doping in conductive electrode material such as activated carbon as well as conducting polymers such as polyaniline or derivatives of polythiophene covering the surface of conductive electrode material.
Pseudocapacitance may also be exhibited given an appropriate structure and pore size of the electrodes, via intercalation. The use of carbide-derived carbons (CDCs) or carbon nanotubes /CNTs for electrodes provides a network of very small pores formed by nanotube entanglement. Carbon nanopores with diameters in the range of <2 nm can be referred to as intercalated pores. Solvated ions in the electrolyte can’t enter these small pores. However de-solvated ions that have smaller ion dimensions are able to enter, increasing the ionic packing density and charge storage. Nano-structured carbon electrodes can increase specific capacitance by faradaic H2 adsorption.[4][5][6]
Three types of electrochemical processes giving rise to pseudocapacitance have been utilized in supercapacitors.[7][8] These are:
- redox reactions involving ions from the electrolyte
- intercalation of atoms out of the electrolyte in the layer lattice, and the
- electrosorption, underpotential deposition of hydrogen or metal adatoms in surface lattice sites
The best understood electrode material for pseudocapacitance is ruthenium oxide (RuO
2).[9] Here the pseudocapacitance originates out of coupled reversible redox reactions with several oxidation steps with overlapping potential. The electrons mostly come from the valence orbitals of the electrode material. The electron transfer reaction is very fast, and can be accompanied by high currents.
The electron transfer reaction is described by:
During charging and discharging in this charge-transfer transition, protons are incorporated into or removed from the ruthenium crystal lattice. This originates an electrochemical faradaic storage of electrical energy without any chemical transformation of the ruthenium. The OH groups are deposited on the electrode surface and remain in the Helmholtz layer. Since the measurable voltage from the redox reaction is proportional to the charged state, the behavior of the reaction is like a capacitor and not like a battery, wherein the voltage is largely independent of the state of charge.

The properties of pseudocapacitance can be expressed in a cyclic voltammogram. For an ideal double-layer capacitor the sign of the current changes immediately after reversing the potential and the shape of the voltammetry is rectangular. For this electrostatic type of energy storage the current is independent on potential of the electrode. For double-layer capacitors with resistive losses the shape changes into a parallelogram. For electrodes with pseudocapacitance the charge stored is strongly dependent on the potential of the electrode. Therefore the voltammetry characteristics deviate from the parallelogram, caused by a delay of potential during the reversal, coming from kinetic processes during charging.[2][11]
References[edit]
- ^ Cite error: The named reference
Halperwas invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
Frackowiak1was invoked but never defined (see the help page). - ^ Garthwaite, Josie (12 July 2011). "How ultracapacitors work (and why they fall short)". Earth2Tech. GigaOM Network. Retrieved 23 April 2013.
- ^ Pandolfo, A. G.; Hollenkamp, A. F. (2006). "Carbon properties and their role in supercapacitors". Journal of Power Sources. 157: 11. doi:10.1016/j.jpowsour.2006.02.065.]
- ^ B.P. Bakhmatyuk, B.Ya. Venhryn, I.I. Grygorchak, M.M. Micov and S.I. Mudry, INTERCALATION PSEUDO-CAPACITANCE IN CARBON SYSTEMS OF ENERGY STORAGE [1]
- ^ P. Simon, A. Burke, Nanostructured carbons: Double-Layer capacitance and more [2]
- ^ B.E. Conway, W.G. Pell, Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid devices, [3]
- ^ B. E. Conway, V. Birss, J. Wojtowicz, Journal of The role and the utilization of pseudocapacitance for energy storage by supercapacitors, Journal of Power Sources, Volume 66, Issues 1–2, May–June 1997, Pages 1–14 [4]
- ^ Cite error: The named reference
conway1was invoked but never defined (see the help page). - ^ Simon, P.; Gogotsi, Y. (11/2008). "Materials for electrochemical capacitors" (PDF). 7. Nature Materials.
{{cite journal}}: Check date values in:|date=(help); Cite journal requires|journal=(help) - ^ Why does an ideal capacitor give rise to a rectangular cyclic voltammogram (CV)? [5]
Fair use candidate from Commons: File:Activated-carbon.jpg[edit]
The file File:Activated-carbon.jpg, used on this page, has been deleted from Wikimedia Commons and re-uploaded at File:Activated-carbon.jpg. It should be reviewed to determine if it is compliant with this project's non-free content policy, or else should be deleted and removed from this page. If no action is taken, it will be deleted after 7 days. Commons fair use upload bot (talk) 23:25, 14 June 2013 (UTC)
HT supercap[edit]
Here's some new info. Don't know where to put it: [9]
- Lfstevens (talk) 17:53, 5 September 2013 (UTC)
- Hi, it is either an additional information under "Electrolyte", keyword: 200 °C electrolyte, or under new developments: Keyword: Supercaps with new RTIL electrolyte for 200 °C applications. As far as I know is this electrolyte not a complete new developed electrolyt, otherwise the authors "forget" to mention, that for tantalum electrolytic capacitors with manganese electrolyte the 200 °C are available since decades. --Elcap (talk) 07:58, 6 September 2013 (UTC)
Silicon supercap[edit]
Check this out: [10] and Oakes, L.; Westover, A.; Mares, J. W.; Chatterjee, S.; Erwin, W. R.; Bardhan, R.; Weiss, S. M.; Pint, C. L. (2013). "Surface engineered porous silicon for stable, high performance electrochemical supercapacitors". Scientific Reports. 3. doi:10.1038/srep03020. Lfstevens (talk) 01:45, 25 October 2013 (UTC)
- Hi Lfstevens, the American researcher are very clever in finding new words for electronic components. Here the silicon only is the mechanical basic of a collector with carbon coating. It is a special type of a carbon electrode. The resulting capacitor belongs to a range of "embedded" capacitors in integrated circuits. The energy density is comparable with standard types of supercapacitors, see table. The power density is much lower than other types of embedded capacitors f. e. with "high-k-dielectric". Greetings --Elcap (talk) 09:02, 27 October 2013 (UTC)
Here's another new thing: powdered graphene at 64 wh/kq
- Hi, this new development reaches energy densities in the range of other new developments. I can't judge, wether the practical realisation really can reach such high values. Lets wait and see. --Elcap (talk) 14:29, 17 November 2013 (UTC)
Device or component[edit]
Supercaps, like all other capacitors, resistors and so on are electric or electronic components, not devices. Lfstevens, please change back all "devices" into components --Elcap (talk) 10:18, 13 January 2014 (UTC)
Copyediting again[edit]
Grinding away. Feedback encouraged. Comments:
- Mention the typical power density of a traditional capacitor for comparison.
- Since the section on pseudocapacitance is much stronger than the article on it, I propose shortening the section and moving the rest of the content to the article. I did not make that change.
- Why are composite electrodes linked to hybrid capacitors? The definition doesn't seem to fit.
- Composite electrodes don't seem to fit under the Hybrid head. They seem to require their own l3 head. I did not make this change. It would be great to add a diagram to match those for EDLC and pseudo electrodes.
- The section on voltage does not clearly distinguish rated voltage from limited voltage or explain why the voltage for a single electrode is interesting.
- Describe whether/why electrolyte cannot be added to a failing component to restore it to full power.
- There are still redundancies. I'll be back one day.
Cheers! Lfstevens (talk) 22:37, 14 January 2014 (UTC)
Hi Lfstevens, "introduction", I turn something back, here the reasons:
- Supercapacitors (SC), are a family of electrochemical capacitors. They are also called ultracapacitors or electric double-layer capacitor (EDLC) or pseudocapacitors. >>>> The former EDLC now are coined SC, because now we know of the both double layer and pseudocapacitance unknown in the 1960 coining the term EDLC. Ultracapacitor is the trade name of ECLC from Maxwell. Articles coming out of the Maxwell corner are talking about Ultracaps, all other say Supercap. I turn back to my introduction.
- Supercapacitors differ from their traditional ancestors... >>>> No, they don’t “differ”, the real electrochemical function was unknown in the past.
- …of a conventional solid dielectric (a barrier that prevents charges from passing from one electrode to the other) >>>> this is not the place for an explanation of the dielectric.
Other abridgements are welcome. --Elcap (talk) 13:04, 15 January 2014 (UTC)
Energy and power[edit]
I corrected "specific energy" and "energy density" to conform with standard definitions (as appear elsewhere in Wikipedia). However, power still has problems. Also, I'm concerned about using "W" to mean energy in the formulas. Work usually refers to moving a body by applying force; recommend using "E". 3dimen (talk) 06:22, 18 October 2014 (UTC)
- @Narky Blert: I think this subject has been addressed before by 3dimen. Some of the uses of specific power or specific energy in the article seem either sloppy or peculiar.
- (BTW: shouldn't one link be sufficient?) —jmcgnh(talk) (contribs) 20:03, 16 August 2016 (UTC)
Lead[edit]
The lead has had a 'lead rewrite' banner since March 2014. I reworked the lead moving much of the detail into the 'Basics' section a few hours ago and removed the banner. The lead has since been reinstated to very much the same content as before (minus the banner). I have reinstated the banner. I note however that we now have verbatim repeated content in the lead and at in 'Basics' section. How can we move forward on this? Fyi, I worked on the lead in the belief that it was more important for the lead to describe there characteristics and say why they were useful than to go into huge detail about how they were manufactured. Happy to engage with this over the next few days, but the current situation seems unsatisfactory. PeterEastern (talk) 09:37, 17 December 2014 (UTC)
- Hi Peter,yes, the lead has had a 'lead rewrite' banner since March 2014. I never take care of that because I thought “quality goes for (lesser) quantity”. Let me give you an explanation what happened with this article. First the description belongs to “electric double-layer capacitor”. But since Brian Evans Conway described the pseudocapacitance it is not correct anymore to describe this component only with double-layer capacitance. Depending on electrode material and surface structure every formerly called EDLC has both different capacitances. So some Wikis asked me to change the title and the text into “Supercapacitor” to describe a capacitor having double-layer capacitance AND pseudocapacitance. (please see above: Change in understanding: From Electric double-layer capacitor to Supercapacitor). For me the banner was something from someone who never understand the new scientific situation. If you can shorten the text without eliminate arguments – let’s try it. But please understand, it is a new description based on new scientific knowledge of this (very complicated) capacitor.
- (Please excuse my “Denglish”). Best regards --Elcap (talk) 15:12, 17 December 2014 (UTC)
- Thank you for responding. I worked on the lead because I agreed with whoever it was who put the banner in place and felt that the existing one was too complicated for the general reader. WP guidance suggests that the lead should start with an opening para that should 'define the topic with a neutral point of view, but without being 'overly specific'. (MOS:INTRO). That the lead should be no more than four paragraphs long in total (WP:LEADLENGTH). That citations should not normally be presented in the lead (WP:CITELEAD). Sorry if I come across as a wikilawyer, that is not my intention, but I am intending to lay out the likely reasoning behind the original banner and provide links to articles which explain the reasoning behind the policies. PeterEastern (talk) 16:09, 17 December 2014 (UTC)
- Hi Peter, that’s the Wiki way of thinking – in paragraphs. I prefer the common way of explaining: The most important informations are a must. If you describe the theme “Beer” in an article and you only write about the taste and don’t mention the composition of alcohol and water something goes wrong. If you want to explain the supercapacitor without explaining the composition of different charge systems something goes wrong. Sometimes the real world is more than common paragraphs wants. I don’t see an “overlay specific”, I only can see wrong simplifications. Greetings --Elcap (talk) 16:32, 17 December 2014 (UTC)
- With respect, I do think it would be appropriate to review the lead in light of the policy guidance that I have linked to above. Would it not be appropriate to cut the lead down to four paragraphs and remove the citation links from the lead as suggested? Could you also have another look at the opening para? To quote from MOS:INTRO: 'The subject should be placed in a context familiar to a normal reader'. I also suggest it is best to restrict the opening para to say what it 'is', and not what it 'is not'. As such, I suggest that the sentence 'As opposed to nanoscale dielectric capacitors[2] which also have high capacitance values, supercapacitors don't have a conventional solid dielectric' is unhelpful and should be bumped from the open para, and possibly out of the lead entirely. PeterEastern (talk) 16:44, 17 December 2014 (UTC)
- Hi Peter, I don't know who wrote the sentence As opposed to nanoscale dielectric capacitors[2] which also have high capacitance values,, but in my original draft stood: in contrast to conventional capacitors, which have a dielectric layer, supercapacitors don't have a conventional dielectric layer. I will change it back. By the way, the article describe a electronic component, not a tram. Please delete the (double) picture on this preferred place. Greetings--Elcap (talk) 08:53, 19 December 2014 (UTC)
- With respect, I do think it would be appropriate to review the lead in light of the policy guidance that I have linked to above. Would it not be appropriate to cut the lead down to four paragraphs and remove the citation links from the lead as suggested? Could you also have another look at the opening para? To quote from MOS:INTRO: 'The subject should be placed in a context familiar to a normal reader'. I also suggest it is best to restrict the opening para to say what it 'is', and not what it 'is not'. As such, I suggest that the sentence 'As opposed to nanoscale dielectric capacitors[2] which also have high capacitance values, supercapacitors don't have a conventional solid dielectric' is unhelpful and should be bumped from the open para, and possibly out of the lead entirely. PeterEastern (talk) 16:44, 17 December 2014 (UTC)
- Hi Peter, that’s the Wiki way of thinking – in paragraphs. I prefer the common way of explaining: The most important informations are a must. If you describe the theme “Beer” in an article and you only write about the taste and don’t mention the composition of alcohol and water something goes wrong. If you want to explain the supercapacitor without explaining the composition of different charge systems something goes wrong. Sometimes the real world is more than common paragraphs wants. I don’t see an “overlay specific”, I only can see wrong simplifications. Greetings --Elcap (talk) 16:32, 17 December 2014 (UTC)
- Thank you for responding. I worked on the lead because I agreed with whoever it was who put the banner in place and felt that the existing one was too complicated for the general reader. WP guidance suggests that the lead should start with an opening para that should 'define the topic with a neutral point of view, but without being 'overly specific'. (MOS:INTRO). That the lead should be no more than four paragraphs long in total (WP:LEADLENGTH). That citations should not normally be presented in the lead (WP:CITELEAD). Sorry if I come across as a wikilawyer, that is not my intention, but I am intending to lay out the likely reasoning behind the original banner and provide links to articles which explain the reasoning behind the policies. PeterEastern (talk) 16:09, 17 December 2014 (UTC)
A removed table entry[edit]
Someone named Elcap has removed an important table entry from the 2015 Jan 27 version. In that version, in the New Developments section, the last table entry says: "Quantum nanoclusters of dipolar metal oxides in TiO2 or TAO2, 2013,480 Wh/kg". Just search for "480 Wh/kg" and you will find it. It has three citations.
In the subsequent 2015 Feb 08 edit by Elcap, this table entry is gone. After talking with him on his talk page, it appears to be a mistake, but so far he has not replaced it.
I would like to know a few things: Could someone replace the table entry? Wasn't there a bot that warned users to let people know they did something bad? If not, where is the warn feature?DrZygote214 (talk) 02:32, 17 February 2015 (UTC)
- Hi DrZygote, I have inserted the line "nanocluster" again. It was not me, that deleted this line. I found the text on a version from 7. January 2015. I could not find the user who delete this line. It is now o.k. so ?--Elcap (talk) 14:58, 17 February 2015 (UTC)
- Hi, User:Otutusaus have deleted the quantum nanoclusters again, reason: information from 2003 not from 2013, too old. --Elcap (talk) 16:33, 18 February 2015 (UTC)
Styles and construction details[edit]
The definition of the term "style" is given in the IEC standard 60384-1, Fixed capacitors for use in electronic equipment – Part 1: Generic specification: "Style" is a subdivision of a type, generally based on dimensional factors, which may include several variants, generally of a mechanical order.
A style can be constructed in different ways, flat, wound, stacked.
A style can be small or large. All shown figures in the gallery are "Supercapacitors". --Elcap (talk) 09:20, 26 November 2015 (UTC)
- The lead quite clearly states that a supercapacitor, "is a high-capacity electrochemical capacitor with capacitance values greater than 1,000 farads". Of the illustrations used, one is 1.0F and therefore 1000 times too small to be a supercapacitor, another sows a 40F, 100F and a 200F and so all three are too small to be supercapacitors. The remaining one is not marked, but scaling it from the packaging, it too must be substantially smaller than 1000F.
- In any event the use of galleries of images is highly discouraged. but this case, the point is moot because the images used in the gallery have no connection with this article for the reasons now stated three times. If you wish illustrate styles, then please do so using illustrations of real supercapacitors and do not present them as a gallery. 86.145.215.191 (talk) 17:41, 26 November 2015 (UTC)
- Sorry, I have corrected the figure in the lead from 1,000 F into "roughly 0.1 F". Please see tabe "Comparison of technical parameters". The 1,000F was a mistake. Supercapacitors, formerly called double layer capacitors, cover the range from 0.068 F up to some 1000 F. (I am just busy to check the figures in this 2 years old table and I have found a lot of new informations, however the range from 0.1 F to some 1000 F is not affected)
- You are not right to say the different styles are not important for the sense of this article. Let me give the reasons:
- The flat style is important for flat devices. the single ended style is important for automatic placement in industrial devices and the screw terminal style is necessary for high currents. We decribe a technical product with technical applications and it is really important to see that engineers can play with the styles to develop optimal solutions.
- If you dont like galleries please feel free to place the three figures with the different styles of "real supercapacitors" in single pictures. --Elcap (talk) 08:12, 27 November 2015 (UTC)
- @Elcap:Right: now we are getting somewhere. Unfortunately, the article is still suffering from inconsistency. The lead now says greater than 0.1F, but later in the article it says "a capacitance value in the order of 1 to 100 farad". I don't know where you got your new figure that you put in the lead from but it appears to be your personal knowledge which is forbidden. Whatever the accepted figure is, it must be backed by a verifiable and reliable reference. 86.145.215.191 (talk) 14:18, 27 November 2015 (UTC)
- Hi, sorry that I got only an IP address from you, normally I want to talk with a name, not a number.
- In the time between I wrote the text for this article 2013 up to now a lot of editors have changed a lot. So, maybe, the wrong cap and voltage values came in. I now have added the ranges of tis type of capacitors. This figures are the result of analysing of the datasheets of round 30 manufacturers wordwide. All the figures you can see in the table "Electrical parameter of supercapacitor series of different manufacturers" in the paragraph "Comparisation of supercapacitor parameters". All manufactures have an ref with the internet website so everybody can check, wether the figures are right or wrong. I dont see, that this is a "forbidden" work.
- If you check some of this datasheets of the different manufacturers you may see the different styles of the capacitors. I think it is inportant for a lot of readers to have an idea how different this capacitors can look. This different styles of the "hardware" are in every case result of device design, assembling conditions and manufacturing facilities. However, the pictures I put in into the article are a question of what I have found "for free" to give it "for everybody" into commons. If you have better pictures, you can better the article.--Elcap (talk) 09:51, 28 November 2015 (UTC)
- A number all you are going to get as I don't have an account. We numbers are as entitled to edit Wikipedia as any name. "Wikipedia: the encyclopedia that anyone can edit."
- If you are deriving the claim fram 'analysing of (sic) the data sheets' as you claim then that is not allowed as that is synthesis. You are not permitted to interpret what one or multiple data sheets contain within them, you can only claim what they specifically state. A statement such as, [supercapacitors are capacitors that have] "capacitance values in the range of round (sic) 100,000 µF up to some 1000 F" requires a specific reference that says precisely that. Without such a reference, the claim cannot be permitted to remain in the article no matter how correct your original research or even experience and knowledge suggests. 86.145.215.191 (talk) 13:19, 28 November 2015 (UTC)
Nitrogen[edit]
Ready for this? http://spectrum.ieee.org/energywise/semiconductors/materials/nitrogen-can-triple-energy-capacity-of-supercapacitors Lfstevens (talk) 05:42, 19 December 2015 (UTC)
External links modified[edit]
Hello fellow Wikipedians,
I have just modified one external link on Supercapacitor. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Corrected formatting/usage for http://electrochem.cwru.edu/ed/encycl/art-c03-elchem-cap.htm
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
![]() An editor has reviewed this edit and fixed any errors that were found.
An editor has reviewed this edit and fixed any errors that were found.
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—cyberbot IITalk to my owner:Online 17:05, 4 April 2016 (UTC)
- sourcechecked=true – original URL no longer seems to work, raises a problem in External Links section which will have to be addressed. —jmcgnh(talk) (contribs) 20:16, 16 August 2016 (UTC)
Capacitance per volume or per mass comparison[edit]
It would be useful to have a row of capacitance per volume or per mass in the table: "Performance parameters of supercapacitors compared with electrolytic capacitors and lithium-ion batteries." Right now, the row is just capacitance, which can be increased by just making the capacitor larger. Quicknick5k (talk) 20:15, 24 May 2018 (UTC)
- Not really that useful since you can trade off voltage for capacitance. The specific energy is given, which is a useful figure of merit. Constant314 (talk) 22:14, 24 May 2018 (UTC)
Table on comparing commercial capacitors removed[edit]
Someone removed that table from section 6.12 without editing any of the text pointing to the existence of that table. Not able to fix this right now, or track down the details, but wanted to flag that quickly. Ablaut490 (talk) 22:07, 14 June 2019 (UTC) Am interested in list ( table ) of ultracapacitors on sale too. — Preceding unsigned comment added by 46.34.244.119 (talk) 19:50, 22 December 2019 (UTC)
Hemp battery?[edit]
I found the following which may be added to the article:
https://www.bbc.com/news/science-environment-28770876
https://www.yahoo.com/now/alyi-electric-vehicle-design-embraces-182100446.html
https://www.nbcnews.com/tech/innovation/far-out-hemp-could-power-better-super-batteries-n178741
Victor Grigas (talk) 20:44, 25 December 2021 (UTC)
EDLC acronym definition[edit]
The third paragraph from the top says: Electrostatic double-layer capacitors (EDLCs) whereas the section Types says: Electric double-layer capacitors (EDLC)
Googling these phrases gives 3,650 hits for the Electrostic phrase, but 101,000 hits for the Electric phrase.
As an outsider to the topic area, it seems better to be consistent and just go with Electric double-layer capacitors (EDLC). 128.16.8.185 (talk) 15:03, 26 April 2023 (UTC)
Two “Types” sections[edit]
These should probably be consolidated. 96.255.181.130 (talk) 10:44, 23 November 2023 (UTC)
Images seem to suggest that standalone "pseudocapacitor" is a thing[edit]
The page pseudocapacitance says,
Faradaic pseudocapacitance only occurs together with static double-layer capacitance. Pseudocapacitance and double-layer capacitance both contribute inseparably to the total capacitance value.
However, the way the following images are laid out seem to suggest that there's such a standalone thing called a "pseudocapacitor", something with only pseudocapacitance.
These images are uploaded as a family of images by Elcap, someone who's a major contributor and no doubt have much more knowledge in this field than I do. Maybe something can be changed with these pictures? Artoria2e5 🌉 15:30, 4 January 2024 (UTC)
- Yeah... In earlier (June 2013) discussions Elcap had indeed said that Pc-tors cannot be purely based on Pc-tance. Maybe we just change the phrasing a bit on those boxes.
- But this does call into another question: is "hybrid" being defined correctly here? Every "pseudocapacitor" (in the sense of a capacitor with mainly pseudocapacitance) should have a combination of both Helmholtz and Faradaic storage, so won't they all be "hybrid" in this sense? I see that asymmetry is noted as some sort of distinguishing characteristic, but why does it matter and how does it become required by the capacitor mechanism? Artoria2e5 🌉 15:44, 4 January 2024 (UTC)
- PMID 36296898 figure 1 suggests that asymmetry is indeed the defining characteristic of a "hybrid" SC, and that symmetric pseudocapacitors can exist? But simultaneously, doi:10.1016/j.rechem.2023.100885 figure 3 shows… I’ve got no idea how to place it, honestly. Artoria2e5 🌉 16:01, 4 January 2024 (UTC)
- doi:10.1002/smll.202002806 manages to make much more sense. Artoria2e5 🌉 16:08, 4 January 2024 (UTC)
- B-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Technology
- B-Class level-5 vital articles
- Wikipedia level-5 vital articles in Technology
- B-Class vital articles in Technology
- B-Class energy articles
- Mid-importance energy articles
- B-Class electronic articles
- Mid-importance electronic articles
- WikiProject Electronics articles
- B-Class Technology articles
- WikiProject Technology articles
- Articles copy edited by the Guild of Copy Editors






