Talk:Stacking (chemistry)
| This article is rated C-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Daily pageviews of this article
A graph should have been displayed here but graphs are temporarily disabled. Until they are enabled again, visit the interactive graph at pageviews.wmcloud.org |
merge notice: this article definition indentical to that of Stacking (chemistry) V8rik 21:44, 4 June 2006 (UTC)
This article is badly written, filled with factual and grammatical errors, I will hopefully return in a few days to tidy it up
Stacking and pi-pi[edit]
I don't have free full-text access to this article at home : Angew Chem Int Ed Engl. 2008;47(18):3430-4. , but it seems to suggest that the pi-pi interaction description here is inaccurate. The arguments make sense to me (that Coulombic attraction between CH and pi are more responsible). Word is that the author quotes wikipedia as a source of the disinformation. It would be nice if people who saw litter were more inclined to pick it up than complain about it, but that's probably asking too much, especially if the author can use it to make their paper more noteworthy. I'll see if I have the free-text to this via work, and if so consider fixing it after reading it. As I've got the memory of a goldfish, I will probably forget, hence this note. 81.187.202.205 (talk) 22:52, 23 July 2009 (UTC)
Okay, I've had a look at the citation above, it looks good and the consequence is essentially that this whole article is screwed. I'll sort it out during my vacation, at least to the extent that it's not explicitly rubbish. According to the article, well supported by modelling in my judgement, much of stacking effect comes from Van De Waals interactions as is evidenced, for example, by similar energies from stacking cyclohexane and benzene. pi-pi interactions it seems can contribute to stacking, but only in certain (non-typical) circumstances. For example, it's umlikely to be a major contributor to the stacking effect in nucleobase (biological) circumstances. This is not yet reflected in most of the secondary literature. Obviously, there shouldn't be excessive weight to this one piece, but we need to explicitly unconfuse stacking from pi-pi and mention the two approaches ("orthodoxy" vs "recent research suggests"). This would have been a whole lot easier with Open Access, grumble grumble, Wiley, grumble grumble. 131.111.21.21 (talk) 13:30, 24 July 2009 (UTC)
Started rewrite by adding initial section. Haven't got to rewriting rest of article yet, just put in an attention of experts box. 81.187.202.205 (talk) 02:38, 25 July 2009 (UTC)
- This pi-pi disinformation has been repeated in umpteen biochemistry textbooks over the yearsbut constant repetition does not make it true. The electrostatic basis of stacking is discussed at length in the text "Principles of Nucleic Acids" by Wolfram Saenger, Springer 1984 ISBN0387907629;ISBN0387907610 (pbk.) I no longer have my copy so I can't quote pages.96.54.53.165 (talk) 00:51, 22 December 2009 (UTC)
Has this article been abandoned? I just stumbled upon this page, and it drew my attention that the authors were going to re-write the article (a year ago?). For whoever continues to work on this article, please be careful in adapting the article to a single source (or only a few sources). For example, based on Stefan Grimme's 2008 article, the article says "Electrostatic forces actually considerably weaken this effect in aromatics" and "Therefore DNA nucleobases (having one or two rings) probably do not significantly stabilise DNA's stacked structure as a result of their aromaticity" The article you cited, though, is limited to non-charged aromatic arenes (where charges are small), and it also used "classical electrostatics" in the energy decomposition, which I believe means it treated each atom in the molecule as a point charge (so quadrupoles were omitted). Figure 2 shows a ~3-4 kcal/mol improvement in interaction energy with only 3 rings (just like nucleic acid base pairs). The article by Grimme is a good article in a very reputable journal, but the conclusion is that the "pi-stacking effect" is caused by "nonlocal electron correlations between the pi electrons." Anyway, I wanted to pitch in a word of encouragement that the article isn't "screwed." It just needs a few more references to round out the perspective. Hopefully someone will have time to pick this back up soon. 11:53, 11 June 2010 (jf) —Preceding unsigned comment added by Phaethonfire (talk • contribs)
There are definitely some problems with this article. I recently looked at the latest literature on this, and it is certain there is something special about stacking pi systems together. Here are some slides on what I figured out. Eugene Kwan (talk) 03:49, 22 November 2010 (UTC)
You cannot change an entire article because of one reference from one investigator -- it is a biased vantage point. Furthermore, there is zero experimental evidence for the conjecture that pi-pi are not critical forces in DNA base stacking. In other words, do an experiment in which the nucleotides are altered to have less aromaticity and get back to us on that. In any case, the haphazard editing here is kind of crazy. To re-write the topmost paragraph around a single source is reckless imho. I also find it hard to believe that there is a sudden shift energetically from a VDW type interaction to a pi-pi in larger ring systems. Is the author saying this is some sort of discontinuous function and pi-pi only suddenly occurs in larger ring systems. Sounds a little unbelievable that such a complex system is purely not using pi-pi aromatic interactions. At best the writing needs improvement to expand upon the nuances. I imagine the original article more artfully danced around this issue that was presented here. —Preceding unsigned comment added by 128.32.166.159 (talk) 23:53, 25 January 2011 (UTC)
UV absorbance[edit]
UV absorbance by the aromatic rings in DNA is suppressed due to stacking. (Compare UV absorbance of denatured DNA versus dimerised DNA.) Would Van der Waals forces suppress this? Or pi-pi interactions? 76.120.195.172 (talk) 06:44, 7 December 2010 (UTC)
More Comments[edit]
Impressions: The article is reasonably organized, but is lacking in a significant number of details and diagrams.
Suggestions:
- How about some plots of preferred orientations and distances in the PDB? Calculations? Figures for high-level geometries of stacked dimers?
- A figure would go a long way towards explaining the Houk/Wheeler, Hunter/Sanders, Sherill, etc. models. Schemes for the torsion balances and other examples you mention would also be useful.
- I like Grimme's work. That should also get a scheme. Maybe you could comment on which computational methods are appropriate for studying these interactions.
- What are the implications of the pi-stacking phenomenon, both for chemistry and biology? I think that at least in catalysis, it's much more complicated than one would like. Rather than having one stacking interaction that is clearly discernible and responsible for selectivity, it is but one of many factors. Are there specific reactions in which pi stacking is implicated? How important is it for protein folding? Ligand-receptor interactions?
- Some comments regarding the energetics of the interaction would be useful. Is having more "pi-surface" cooperative in any way? If it is a purely dispersive interaction, is it likely to increase in the transition state? At least for cation-pi interactions, that seems likely. Here, it's not so clear.
Eugene Kwan (talk) 22:05, 24 October 2011 (UTC)
A minor but important change. Added SI units (kJ) keeping the older units in parentheses (multiplied simply by 4 since the original values were only approximate). However, I feel strongly that SI units must be used as the primary. — Preceding unsigned comment added by 109.148.69.36 (talk) 09:18, 8 May 2012 (UTC)
I have to disagree. kcal are standard. kJ may be insisted on in rule books, but generally nobody uses them in this context. Went to a talk by Grimme last week, and he used them extensively. Eugene Kwan (talk) 10:37, 8 May 2012 (UTC)
Not where I am .... And most certainly not in major journals. For instance the NEJM insists that SI units be used for all clinical chemistry measurements. All major text books use SI units - my very elder copy of Lehninger (Second edition) uses Joules as does my copy of Metzler. The use of kcal outside the US is virtually unheard of (and remember wiki is international). — Preceding unsigned comment added by 31.52.122.223 (talk) 19:22, 10 May 2012 (UTC)
No... hartrees are more common ;)
Kilocalories and kilojoules are both used for describing thermochemistry (computationally or experimentally). Perhaps kcal is favored in the US, but I won't go so far as to say that it predominates. It is closer to picometers versus angstroms, than a case of milliliters versus cubic centimeters. In any case, a calorie is still an SI-derived unit. I personally find kcal convenient, because the bond dissociation energies tend to be around 100 kcal/mol.
Anyway, kcal is no more standard than kJ is. If you are reading this article, you probably will know the conversion anyway. As long as we stay consistent, we'll be fine. --Rifleman 82 (talk) 20:56, 10 May 2012 (UTC)

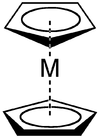
Would the linked-title to this talk-section I added above constitute this kind of pi-stacking: i.e. transition metal chelate eta-six co-ordination? (cf. so-named, systematic S. Singh # 21bd) 66.96.79.217 (talk) 01:50, 21 April 2016 (UTC)
- As far as I can tell from that article and your question (which could be clearer) the benzene and cyclopentadienyl in this cocaine analog are practically on opposite sides of the molecule (I'll admit I'm slightly unclear on what they mean about the solid cone angles). So far as I can tell, your question is almost the same as asking whether a generic metallocene is a case of pi stacking... but I could be wrong. Now you can have stacked metallocenes, but I think what they usually have in mind is something like this with the two aromatic rings together. When they're on opposite sides of a metal, they may be interacting somehow via the multilobed orbitals on the metal, but I don't think that counts as stacking. I'm not that well informed in these things, so if you want a better answer, go to WP:Reference desk/Science - but if you're asking whether you should add that cocaine analog as an illustration of stacking for this article, I'm just barely confident enough to vote no. Wnt (talk) 12:44, 21 April 2016 (UTC)
Rethinking the term “pi-stacking”[edit]
I'm going to leave this citation here.[1] There is a lot of misinformation online and I would hope that Wikipedia might be a means to get through that, even in scientific circles. Fix this page please!
References
- ^ Martinez, Chelsea R.; Iverson, Brent L. (2012). "Rethinking the term "pi-stacking"". Chemical Science. 3 (7): 2191. doi:10.1039/C2SC20045G.
- Nonetheless, the term pi-stacking has been widely adopted in scientific literature and remains in use. I see no reason to strictly depart from these sources. TheBartgry (talk) 10:07, 21 September 2020 (UTC)
- The term “pi-stacking” is widely missued as Martinez and Iverson make clear. Calculations show that the face-to-face orientation is electrostatically unfavorable. Furthermore, the avaiable experimental data (e.g., crystal structures) support that the staggered orientation is far more common than face-to-face orientation. The "evidence for" section of this article is actually "evidence against". I have revised the lead and the "evidence" section heading to make that clear. Boghog (talk) 10:20, 21 May 2022 (UTC)

