Talk:Fluorine compounds
| This subarticle is kept separate from the main article, Fluorine, due to size or style considerations. |
| This article is rated B-class on Wikipedia's content assessment scale. It is of interest to the following WikiProjects: | |||||||||||
| |||||||||||
Compounds (holding pen for content being cut from parent)[edit]
Fluorine's common oxidation state is −1.[note 1] Because of its attraction for electrons, fluorine forms polar covalent bonds or ionic bonds to other atoms. Covalent bonds involving fluorine atoms are almost always single bonds,[note 2][3] although fluorine may act as a bridging ligand between metals in some metal complexes. Molecules containing fluorine may also exhibit hydrogen bonding.[4] Fluorine has a rich chemistry including compounds formed with hydrogen, metals, main group nonmetals, and even noble gases, as well as a diverse set of organic compounds.[note 3][5]
Hydrogen fluoride[edit]
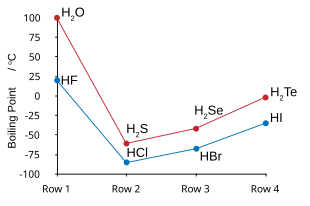
Fluorine combines with hydrogen to make a compound called hydrogen fluoride (HF) or, in the context of water solutions, hydrofluoric acid. HF molecules cluster together weakly via hydrogen bonds. Because of this, hydrogen fluoride behaves more like water than like HCl (hydrochloric acid).[6][7][8] Hydrogen fluoride boils at a much higher temperature than the heavier hydrogen halides. HF is also fully miscible with water (dissolves in any proportion), unlike HCl, HBr, or HI.[9]
In water solution, hydrogen fluoride is a weak acid; the other hydrohalic acids are all strong.[10][note 4] Hydrofluoric acid is non-ideal: instead of having a constant acid dissociation constant (PKa), HF's inherent acidity increases at higher concentrations through a phenomenon called homoconjugation.[12][13] Although hydrofluoric acid is weak, it is very corrosive, even attacking glass.[12]
Metal fluorides[edit]
Metal fluorides share some similarities with other metal halides but are more ionic, often more like oxides in their bonding and crystal structures.[14] However, there is a trend of the binary metal fluorides becoming more covalently bonded and volatile as the amount of fluorination increases. For example, sodium monofluoride boils at 1704 °C,[15] whereas rhenium heptafluoride has a boiling point below water's.[16] Similar to fluorine itself, the higher metal fluorides are very reactive. Platinum hexafluoride was the first compound to oxidize molecular oxygen[17] and xenon.[18] The solubility of fluorides varies greatly but tends to decrease with more fluorine.[19]
The alkali metals form monofluorides that, like the alkali metal chlorides, are very ionic and soluble. They have the same atomic arrangement—the rock salt crystal structure—as sodium chloride.[20][21] Other metal monofluorides show clear difference versus their chlorides. For example, thallium[22] and silver[23] monofluorides are soluble, while the chlorides are not.
The difluorides of the alkaline earths are also very ionic, but are generally very insoluble. In contrast, the alkali earth dichlorides are fairly soluble.[23] Beryllium's difluoride differs from those of the other alkaline earths: the bonding has some covalent character, the structures are similar to SiO2 (quartz), and the compound is water soluble.[24]
Many metals form trifluorides, including iron, bismuth, the rare earths, some actinides, and the metals in the aluminium and scandium columns of the periodic table. In general, the metal trifluorides have ionic bonding structures with octahedral coordination of the metal cations. Some show the same structure as rhenium trioxide. The larger metal atoms can have dense structures with more than six surrounding fluorines. In contrast, gold's trifluoride has an open structure with helical chains of square planar AuF4 units.[25][26][27]
The tetrafluorides show a mixture of ionic and covalent bonding. Zirconium[28] and hafnium,[29] along with several actinides,[30] form tetrafluorides with an ionic structure based on 8-coordinate metal cations.[31] Melting points are around 1000 °C.[note 5] On the other hand, the tetrafluorides of titanium,[34], vanadium,[35] niobium[36] are polymeric. The first melts at 284 °C;[37] the latter two decompose at around 350 °C.[38]
The pentafluorides are even more covalently bonded, forming low dimensionality polymers or oligomeric molecules.[39] Bismuth and uranium pentafluorides share the same structure: straight chains of connected octahedra.[40][41][42] Gold's pentafluoride is a dimer (doubled molecule), Au2F10.[39] Niobium and tantalum pentafluorides are tetrameric molecules (fourfold clusters).[39] The former melts at 80 °C,[43] the latter at 95 °C.[44]
A total of thirteen metal hexafluorides have been characterized.[note 6] All are octahedral molecules.[45] At room temperature tungsten hexafluoride is a gas.[33] Molybdenum hexafluoride and rhenium hexafluoride are liquids.[46] The rest are volatile solids.[47]
The only definite metal heptafluoride, that of rhenium, is a low-melting molecular solid. The structure is a distorted pentagonal bipyramid: the five equatorial fluorines are buckled slightly above and below the plane.[14] In 1966, osmium heptafluoride was reported from daunting synthesis conditions (400 atmospheres of fluorine gas at 600 °C), but the product was not structurally characterized. In 2006, the synthesis was re-attempted but only osmium hexafluoride was observed.[48] Previously, a 1913 report of an octafluoride, that of osmium, was shown in 1958 to be a mistaken identification of the same compound, osmium hexafluoride.[5][49]
| Progression of structure type with metal charge in the metal fluorides | ||

|
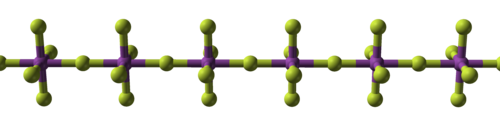
|

|
| Sodium fluoride, ionic | Bismuth pentafluoride, polymeric | Rhenium heptafluoride, molecular |
Nonmetal fluorides[edit]
The binary fluorides of the main group nonmetals and metalloids are generally volatile, covalently bonded molecules. Except for boron, the elements in the second row of the periodic table (carbon, nitrogen, and oxygen) form fluorides that follow the octet rule. Nonmetals from the third row of the periodic table and below can form fluorides which are hypervalent (more bonds than normal).[50]
Boron trifluoride is a planar molecule where the boron atom has an incomplete octet (less bonds than normal). It is a weak Lewis acid and readily accepts a Lewis base, forming adducts (combinations). The lone pair of a Lewis base, such as ammonia, allows the boron to complete its octet. With another fluoride ion (F− is a Lewis base), BF3 reacts to form the relatively unreactive BF−
4 anion.[51]
The simplest binary compound with carbon is carbon tetrafluoride, an inert tetrahedral molecule.[note 7] Silicon tetrafluoride and germanium tetrafluoride are also tetrahedral[52] but are Lewis acids.[53][54] Silicon forms many polysilicon fluorides (even more diversity than the silanes). With excess silicon, gaseous SiF2 is made, which condenses into a polymer. Forms of SinF2n+2 are liquids and solids and are known up to Si14F30.[55] Reaction of excess germanium with its tetrafluoride also creates a difluoride. The compound is a low-melting solid with spiral chains of linked GeF3 units. Germanium also forms a mixed valence compound (i.e. with both +4 and +2 Ge atoms): [(GeF2)4GeF4].[56]
The pnictogens (nitrogen's column) form trifluorides that are weak Lewis bases and are more reactive as the pnictogen becomes heavier.[57] However, nitrogen trifluoride is stable against hydrolysis and is not a Lewis base.[57] In comparison to the trifluorides, the pnictogen pentafluorides are even more reactive.[58][59] Antimony pentafluoride is the strongest Lewis acid of all charge-neutral compounds.[59] The trifluorides have a trigonal pyramid structure (ammonia's shape). The pentafluorides are generally trigonal bipyramidal. However, the liquid and solid phases of SbF5 have more complicated structures with octahedral antimony and bridging fluorines.[40]
The chalcogens (oxygen's column) form a variety of fluorides. Unstable difluorides are known for oxygen (the only compound where oxygen is at formal oxidation state +2) as well as sulfur and selenium. The geometry is bent (similar to water). Sulfur and selenium tetrafluorides show the rarece seesaw molecular geometry, but solid TeF4 is a polymer. All the tetrafluorides are thermally unstable and hydrolyze. The hexafluorides are the result of direct fluorination of sulfur, selenium, and tellurium and are octahedral molecules. SF6 is extremely inert, while SeF6 and TeF6 show increasingly higher reactivity. Various chalcofluoride molecules exist with two chalcogen atoms also: O2F2 ("FOOF"), S2F10, etc.[60][61]
The well-characterized heavier halogens (chlorine, bromine, and iodine) all form mono-, tri-, and pentafluorides: XF, XF3, and XF5. Of the neutral heptavalent species, only iodine heptafluoride is known.[62] The corresponding cations, ClF+
6 and BrF+
6, are known and are extremely strong oxidizers.[63] For the radioactive element astatine, only the non-volatile astatine monofluoride has been studied,[64] but its existence is debated.[65] Many of the halogen fluorides are powerful fluorinators (sources of fluorine atoms). ClF3 readily fluorinates asbestos and refractory oxides, and industrial use requires precautions similar to those for fluorine gas.[66][67]
| Notable nonmetal fluorides, across the periodic table columns | ||||

|

|

|

| |
| Boron trifluoride | Silicon tetrafluoride | Antimony pentafluoride | Sulfur hexafluoride | Chlorine trifluoride |
Noble gas compounds[edit]

The noble gases are generally non-reactive because they have complete electron shells. Until the 1960s, no chemical bond with a noble gas was known. In 1962, Neil Bartlett reported the first chemical compound of xenon, xenon hexafluoroplatinate.[68] Later in 1962, xenon was reported to react directly with fluorine to form the di- and tetrafluorides. Since then, xenon hexafluoride, various oxyfluorides, and their derivatives have been prepared.[69][70]
Krypton, xenon's lighter homolog, also forms a difluoride and a few more complicated fluorine-containing compounds.[71] The possibility of a tetrafluoride[72] or a hexafluoride has been debated.[73]
Radon, xenon's heavier homolog, has been shown to readily react with fluorine to form a solid compound. It is generally thought to be radon difluoride, but the exact formula is not known.[64] If radon were not so radioactive and difficult to collect, its chemistry might be at least as extensive as xenon's.[74]
The lightest noble gases do not form stable binary fluorides. Argon, however, reacts in extreme conditions with hydrogen fluoride to form argon fluorohydride.[75] Helium and neon do not form any time-stable fluorides, but helium fluorohydride has been observed for milliseconds at extremely high pressure and low temperature.[76] Neon is considered even less reactive than helium and no fluorides have been even momentarily observed.[77]
Organic compounds[edit]
The carbon–fluorine chemical bond is the strongest bond in organic chemistry.[78] This C–F bond strength, along with the low polarizability of molecules containing C-F, makes organofluorines very stable.[79] Fluorinated organics have similar sizes to corresponding unfluorinated molecules because of the small van der Waals radius of fluorine.[79]
The range of organofluorine compounds is diverse, reflecting the inherent complexity of organic chemistry. A vast number of small molecules can exist with varying amounts of fluorine substitution, as well as many polymers. Research in particular areas is driven by the commercial value of applications.[80]
Small molecules[edit]

Partially fluorinated alkanes are hydrofluorocarbons (HFCs). Monofluoroalkanes (alkanes with one hydrogen replaced with fluorine) have properties similar to unfluorinated alkanes. They are soluble in many nonpolar solvents and have some chemical and thermal instability. As more fluorines are substituted for hydrogens, the properties change. Solubility in hydrocarbons decreases and stability increases. Also, melting and boiling points decrease, while density goes up.[81] When all hydrogens are replaced with fluorines to make perfluorocarbons (the "per" means maximum), a great difference is revealed. Such compounds are extremely stable, and only sodium in liquid ammonia attacks them at standard conditions. They are also very insoluble, with few organic solvents capable of dissolving them.[81]
Perfluorinated compound is a term for hydrocarbons that are fully fluorinated which also have a functional group.[note 8][83] They exhibit many perfluorocarbon properties (e.g. inertness, stability, non-wetting by water and oils, slipperiness).[84] However, the functional group is available for reactions or may make the molecule behave as a surfactant.[85] If a perfluorinated compound has a fluorinated tail but also a few non-fluorinated carbons (typically two) near the functional group, it is called a fluorotelomer. Industrially, such compounds are treated as perfluorinated.[84]
Fluorinating an organic acid raises its acidity. For example, acetic acid and its mono-, di-, and trifluoroacetic derivatives show a trend of lowering pKa: 4.74, 2.66, 1.24, 0.23 (thus increasing acidity).[86] This happens because of fluorine's inductive effect (it stabilizes anions by spreading negative charge, allowing the hydrogen to be released).[87] Similarly, the acidity is greatly increased for other perfluorocarboxyl acids, as well as the amines (which are not acids but become less basic if fluorinated).[79] The perfluoroalkanesulfonic acids are also very notable for their acidity. Trifluoromethanesulfonic acid, is comparable to strong mineral acids.[88][88]
Polymers[edit]

As with small molecules, replacing hydrogen with fluorine in a polymer increases chemical stability and reduces flammability. Melting points are typically much higher than in the corresponding hydrocarbon polymers.[89]
The simplest fluoroplastic is polytetrafluoroethylene (PTFE, DuPont brand Teflon), which is a simple linear chain polymer with the repeating structural unit: –CF2–. It has no hydrogens and is the perfluoro analog of polyethylene (structural unit: –CH2–). PTFE has high chemical and thermal stability, as expected for a perfluorocarbon, much stronger than polyethylene. However, its very high melting point makes it difficult to fashion into parts.[90]
Various PTFE derivatives have lower maximum usage temperatures but have the benefit of being more melt-processable. FEP (fluorinated ethylene propylene is structurally similar to PTFE but has some fluorines replaced with the –CF3 groups). PFA (perfluoroalkoxy has some fluorines replaced with –OCF3).[90] Nafion is a structurally complicated polymer. It has a PTFE-like backbone, but also contains side chains of perfluoro ether that end in sulfonic acid (–SO2OH) groups.[91][92]
There are other fluoroplastics that are not perfluorinated (contain some C-H). Polyvinylidene fluoride (PVDF, structural unit: –CF2CH2–), is an analog of PTFE with half the fluorines. PVF (polyvinyl fluoride, structural unit: –CH2CHF–), contains one one-fourth the fluorines of PTFE. Despite this, it still has many properties of fully fluorinated polymers.[93]
References
- ^ Wiberg, Wiberg & Holleman 2001, p. 422.
- ^ Schlöder & Riedel 2012.
- ^ Harbison 2002.
- ^ Edwards 1994, p. 515.
- ^ a b Riedel & Kaupp 2009.
- ^ Pauling 1960, pp. 454–464.
- ^ Atkins & Jones 2007, pp. 184–185.
- ^ Emsley 1981.
- ^ Greenwood & Earnshaw 1998, pp. 812–816.
- ^ Wiberg, Wiberg & Holleman 2001, p. 425.
- ^ Clark 2002.
- ^ a b Chambers & Holliday 1975, pp. 328–329.
- ^ TM-H 2010, p. 15.22.
- ^ a b Greenwood & Earnshaw 1998, p. 819.
- ^ Lide 2004, p. 4.84.
- ^ Lide 2004, p. 4.79, 4.91.
- ^ Bartlett & Lohmann 1962.
- ^ Bartlett 1962.
- ^ Oxtoby, Gillis & Campion 2012, p. 693.
- ^ Katakuse et al. 1999, p. 267.
- ^ Aigueperse et al. 2005, pp. 25–27.
- ^ Remy 1956, p. 383.
- ^ a b Storer 1864, pp. 278–280.
- ^ Walsh 2009, pp. 99–102, 118–119.
- ^ Emeléus & Sharpe 1983, pp. 89–97.
- ^ Babel & Alain 1985, pp. 91–96.
- ^ Einstein et al. 1967.
- ^ Brown et al. 2005, p. 144.
- ^ a b Perry 2011, p. 193.
- ^ Kern et al. 1994.
- ^ Lide 2004, pp. 4.60, 4.76, 4.92, 4.96.
- ^ Lide 2004, p. 4.96.
- ^ a b Lide 2004, p. 4.92.
- ^ Greenwood & Earnshaw 1998, p. 964.
- ^ Becker & Müller 1990.
- ^ Greenwood & Earnshaw 1998, p. 990.
- ^ Lide 2004, p. 4.91.
- ^ Lide 2004, p. 4.72, 4.93.
- ^ a b c Hwang & Seppelt 2001.
- ^ a b Greenwood & Earnshaw 1998, pp. 561–563.
- ^ Emeléus & Sharpe 1983, pp. 256–277.
- ^ Mackay, Mackay & Henderson 2002, pp. 243–244.
- ^ Lide 2004, p. 4.72.
- ^ Lide 2004, p. 4.88.
- ^ Drews et al. 2006.
- ^ Lide 2004, p. 4.71, 4.78.
- ^ Greenwood & Earnshaw 1998, (various pages, by individual metal in respective chapter).
- ^ Shorafa & Seppelt 2006.
- ^ Weinstock & Malm 1958.
- ^ Noury, Silvi & Gillespie 2002.
- ^ Greenwood & Earnshaw 1998, pp. 375–376.
- ^ Ellis 2001, p. 69.
- ^ Aigueperse et al. 2005, p. 28.
- ^ Wiberg, Wiberg & Holleman 2001, p. 897.
- ^ Greenwood & Earnshaw 1998, pp. 376–377.
- ^ Greenwood & Earnshaw 1998, pp. 198–199.
- ^ a b Raghavan 1998, pp. 164–5.
- ^ Aigueperse et al. 2005, p. 37.
- ^ a b Godfrey et al. 1998, p. 98.
- ^ Murthy, Mehdi Ali & Ashok 1995, pp. 180–182, 206–208.
- ^ Greenwood & Earnshaw 1998, pp. 638–640, 683–689, 767–778.
- ^ Wiberg, Wiberg & Holleman 2001, p. 435.
- ^ Wiberg, Wiberg & Holleman 2001, p. 436.
- ^ a b Pitzer 1993, p. 111.
- ^ Eberle, Berei & Vasáros 1985, p. 224.
- ^ Greenwood & Earnshaw 1998, pp. 828–830.
- ^ Patnaik 2007, pp. 478–479.
- ^ Wiberg, Wiberg & Holleman 2001, pp. 392–393.
- ^ Wiberg, Wiberg & Holleman 2001, p. 438.
- ^ Wiberg, Wiberg & Holleman 2001, p. 400.
- ^ Lewars 2008, p. 68.
- ^ Grosse et al. 2003.
- ^ Dixon 2007.
- ^ Lewars 2008, p. 67.
- ^ Cite error: The named reference
Khriachtchev et al. 2000was invoked but never defined (see the help page). - ^ Bihary, Chaban & Gerber 2002.
- ^ Lewars 2008, p. 71.
- ^ O'Hagan 2008.
- ^ a b c Siegemund et al. 2005, p. 2.
- ^ Cite error: The named reference
Jstgwas invoked but never defined (see the help page). - ^ a b Siegemund et al. 2005, pp. 7–8.
- ^ Posner et al. 2013, pp. +hydrocarbons+that+are+fully+fluorinated+except+for+one+functional+group%22 187–190.
- ^ Barbee, McCormack & Vartanian 2000, p. 116.
- ^ a b Posner 2011, p. 27.
- ^ Salager 2002, p. 45.
- ^ Siegemund et al. 2005, p. 29.
- ^ Bansal 2003, p. 52.
- ^ a b Siegemund et al. 2005, p. 32.
- ^ Carlson & Scmiegel 2005, p. 3.
- ^ a b Carlson & Scmiegel 2005, pp. 3–4.
- ^ Rhoades 2008, p. 2.
- ^ Okada et al. 1998.
- ^ Carlson & Scmiegel 2005, p. 4.
Binary compounds[edit]
I am well aware of argon fluoride lasers. But as a compound, argon fluoride does not count, because it's not termodynamically stable under any conditions.--R8R (talk) 16:24, 1 April 2015 (UTC)
Blacklisted Links Found on Compounds of fluorine[edit]
Cyberbot II has detected links on Compounds of fluorine which have been added to the blacklist, either globally or locally. Links tend to be blacklisted because they have a history of being spammed or are highly inappropriate for Wikipedia. The addition will be logged at one of these locations: local or global If you believe the specific link should be exempt from the blacklist, you may request that it is white-listed. Alternatively, you may request that the link is removed from or altered on the blacklist locally or globally. When requesting whitelisting, be sure to supply the link to be whitelisted and wrap the link in nowiki tags. Please do not remove the tag until the issue is resolved. You may set the invisible parameter to "true" whilst requests to white-list are being processed. Should you require any help with this process, please ask at the help desk.
Below is a list of links that were found on the main page:
- http://www.drugfuture.com/chemdata/vanadium-tetrafluoride.html
- Triggered by
\bdrugfuture\.com\bon the local blacklist
- Triggered by
If you would like me to provide more information on the talk page, contact User:Cyberpower678 and ask him to program me with more info.
From your friendly hard working bot.—cyberbot IITalk to my owner:Online 08:44, 11 September 2015 (UTC)
Tetrafluorine cation?!?!?[edit]
I thought F+
4 doesn't exist. Alfa-ketosav (talk) 19:20, 3 July 2018 (UTC)
- What else? What change to the article do you want to discuss? Plasmic Physics (talk) 20:36, 3 July 2018 (UTC)
To merge[edit]
{{Fluorides}} and {{Fluorine compounds}} should be merged. -DePiep (talk) 21:09, 26 July 2020 (UTC)
Move discussion in progress[edit]
There is a move discussion in progress on Talk:Compounds of aluminium which affects this page. Please participate on that page and not in this talk page section. Thank you. —RMCD bot 05:39, 5 May 2021 (UTC)
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).
