Magnesium permanganate
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider |
|
| ECHA InfoCard | 100.030.740 |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
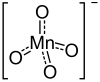
| Mg(MnO4)2 | |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium permanganate is an inorganic compound with the chemical formula Mg(MnO4)2. It can be used as an oxidant.[1]
Preparation[edit]
Magnesium permanganate hexahydrate was prepared by E. Mitserlich and H. Aschoff by reacting barium permanganate with magnesium sulfate:[2]
- MgSO4 + Ba(MnO4)2 → Mg(MnO4)2 + BaSO4
It can be obtained by the reaction of magnesium chloride and silver permanganate:
- MgCl2 + 2AgMnO4 → Mg(MnO4)2 + 2AgCl
The hexahydrate Mg(MnO4)2·6H2O can be crystallized from the solution, which is slightly hygroscopic.[3] The anhydrous form can be obtained by decomposing the hexahydrate by heating it.
Chemical properties[edit]
Magnesium permanganate hexahydrate is a blue-black solid.[4] It decomposes at 130 °C with the evolution of oxygen in an autocatalytic decomposition process. The tetrahydrate decomposes above 150 °C. The crystals are practically insoluble in carbon trichloride, carbon tetrachloride, benzene, toluene, nitrobenzene ether, ligroin and carbon disulfide, but soluble in pyridine and glacial acetic acid. It dissolves in water and dissociates completely in dilute solutions. It oxidizes a range of organic compounds and reacts instantly (in some cases with fire) with common solvents such as tetrahydrofuran, ethanol, methanol, t-butanol, acetone and acetic acid.[2]
Applications[edit]
Magnesium permanganate is used in various branches of industry and technology, such as:[2]
- a wood impregnation agent.
- an additive in tobacco filters.
- as a catalyst in the air oxidation of toluene to benzoic acid and in proteome research.
References[edit]
- ^ Saul Wolfe, Christopher F. Ingold (Dec 1983). "Oxidation of organic compounds by zinc permanganate". Journal of the American Chemical Society. 105 (26): 7755–7757. doi:10.1021/ja00364a054. ISSN 0002-7863. Archived from the original on 2019-10-24. Retrieved 2020-06-27.
- ^ a b c Kotai, Laszlo; Gacs, Istvan; Sajo, Istvan E.; Sharma, Pradeep K.; Banerji, Kalyan K. (2011-03-29). "ChemInform Abstract: Beliefs and Facts in Permanganate Chemistry - An Overview on the Synthesis and the Reactivity of Simple and Complex Permanganates". ChemInform. 42 (13): no. doi:10.1002/chin.201113233.
- ^ Moles, E.; Crespi, M. Permanganates. III. Anales de la Real Sociedad Espanola de Fisica y Quimica, 1923. 21. 305-316. ISSN: 0365-6675.
- ^ Haynes, William M. (2016-06-22). CRC Handbook of Chemistry and Physics. CRC Press. ISBN 978-1-4987-5429-3.
