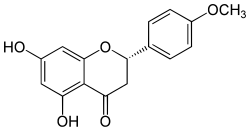
Isosakuranetin
| |
| Names | |
|---|---|
| IUPAC name
(2S)-5,7-Dihydroxy-4′-methoxyflavan-4-one
| |
| Systematic IUPAC name
(2S)-5,7-Dihydroxy-2-(4-methoxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
4'-Methylnaringenin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.866 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H14O5 | |
| Molar mass | 286.27 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isosakuranetin, an O-methylated flavonoid, is the 4'-methoxy derivative of naringenin, a flavanone. Didymin, a disaccharide of isosakuranetin, occur e.g. in sweet orange, blood orange and mandarin.[1] Isosakuranetin is a potent inhibitor of TRPM3 channels.[2]
Glycosides[edit]
- Poncirin is the 7-O-neohesperidoside of isosakuranetin.
- Didymin is the 7-O-rutinoside of isosakuranetin
References[edit]
- ^ "Polyphenols in Human Health and Disease" ISBN 9780123984562
- ^ Straub, Isabelle; Krügel, Ute; Mohr, Florian; Teichert, Jens; Rizun, Oleksandr; Konrad, Maik; Oberwinkler, Johannes; Schaefer, Michael (November 2013). "Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo". Molecular Pharmacology. 84 (5): 736–750. doi:10.1124/mol.113.086843. ISSN 1521-0111. PMID 24006495.
