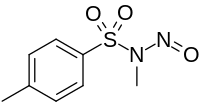
Diazald
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,4-Dimethyl-N-nitrosobenzene-1-sulfonamide | |
| Other names
N-Methyl-N-nitroso-4-methylbenzenesulfonamide; N-Methyl-N-nitroso-p-toluenesulphonamide; N-Methyl-N-nitroso-4-methylbenzenesulphonamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.139 |
| EC Number |
|
| MeSH | C418734 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H10N2O3S | |
| Molar mass | 214.24 g·mol−1 |
| Appearance | Light yellow solid |
| Melting point | 61–62 °C (142–144 °F; 334–335 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Skin sensitiser, irritant, explosive[1] |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Diazald (N-methyl-N-nitroso-p-toluenesulfonamide) is used as a relatively safe and easily handled precursor to diazomethane, which is toxic and unstable.[2] Diazald has become the favored commercially available precursor for the synthesis of diazomethane, compared to reagents like N-methyl-N-nitrosourea and N-methyl-N'-nitro-N-nitrosoguanidine, which are less thermally stable and more toxic and mutagenic, respectively.
Upon the addition of a base such as sodium hydroxide or potassium hydroxide and mild heating (65–70 °C) in a mixture of water, diethyl ether, and a high boiling polar cosolvent (e.g., diethylene glycol monomethyl ether[3]), the N-nitrososulfonamide undergoes successive elimination reactions to produce diazomethane (which is codistilled as an ethereal solution) as well as a p-toluenesulfonate salt as a byproduct, according to the following mechanism:

Like other nitroso compounds, it is thermally sensitive, as a result of its weak N–NO bond whose bond dissociation energy was measured to be 33.4 kcal/mol.[4]
References[edit]
- ^ External MSDS, Sigma Aldrich
- ^ Diazald in Chemical Synthesis, Sigma Aldrich
- ^ "Diazomethane". www.orgsyn.org. Retrieved 2018-07-27.
- ^ Zhu, Xiao-Qing; Hao, Wei-Fang; Tang, Hui; Wang, Chun-Hua; Cheng, Jin-Pei (March 2005). "Determination of N−NO Bond Dissociation Energies ofN-Methyl-N-nitrosobenzenesulfonamides in Acetonitrile and Application in the Mechanism Analyses on NO Transfer". Journal of the American Chemical Society. 127 (8): 2696–2708. doi:10.1021/ja0443676. ISSN 0002-7863. PMID 15725027.

