Datumetine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
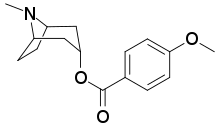
| Formula | C16H21NO3 |
| Molar mass | 275.348 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Datumetine is a tropane alkaloid found in leaves of Datura metel.[1] It is said to modulate NMDA receptor and thus causes memory loss.[2] It also causes epileptic seizures in mice.[2] Docking studies suggest that it fits on both allosteric and orthosteric sites of NMDA receptor.[2] It acts together with other anticholinergic tropane alkaloids of datura to cause amnesia.[citation needed]
See also[edit]
References[edit]
- ^ Siddiqui S, Sultana N, Ahmed SS, Haider SI (2004). "Isolation and Structure of a New Alkaloid Datumetine from the leaves of Datura metel". Journal of Natural Products. 49 (3): 511–513. doi:10.1021/np50045a023. ISSN 0163-3864.
- ^ a b c Ishola AO, Imam A, Ajao MS (2021). "Effects of datumetine on hippocampal NMDAR activity". Toxicology Reports. 8: 1131–1142. doi:10.1016/j.toxrep.2021.05.009. PMC 8190477. PMID 34150523.
