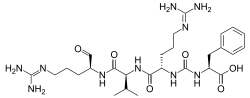
Antipain
| |
| Names | |
|---|---|
| IUPAC name
N2-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}-N5-(diaminomethylidene)-L-ornithyl-N-{(2S)-5-[(diaminomethylidene)amino]-1-oxopentan-2-yl}-L-valinamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H44N10O6 | |
| Molar mass | 604.713 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain.[1] It was discovered in 1972 and was the first natural peptide found that contained an ureylene group.[2] Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases.[3]
It has been crystallized in complexes with carboxypeptidase, which is obtained from wheat,[4] and Leishmania major oligopeptidase B.[5] In both cases, the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease.
A study was performed for information on the effect of antipain on the quality of post-thawed ram semen.[6] The results from this experiment concluded that antipain aided in the quality of ram semen by maintaining the sperm mobility.[6] Antipain includes the function to inhibit a degrading enzyme, called plasmin, permitting this substance to be able to improve the resistance of membrane disruption by freezing temperatures.[6]
Antipain Y, a similar chemical compound that is an analog of antipain, which was isolated from a species of Streptomyces, inhibits the release of neurotransmitters in rat dorsal root ganglion neurons.[7]
References[edit]
- ^ Suda H, Aoyagi T, Hamada M, Takeuchi T, Umezawa H (April 1972). "Antipain, a new protease inhibitor isolated from actinomycetes". The Journal of Antibiotics. 25 (4): 263–266. doi:10.7164/antibiotics.25.263. PMID 4559651.
- ^ Umezawa S, Tatsuta K, Fujimoto K, Tsuchiya T, Umezawa H (April 1972). "Structure of antipain, a new Sakaguchi-positive product of streptomyces". The Journal of Antibiotics. 25 (4): 267–270. doi:10.7164/antibiotics.25.267. PMID 5052959.
- ^ Lackie J (2012). A Dictionary of Biomedicine. Oxford University Press. ISBN 9780199549351.
- ^ PDB ENTRY 1bcr Bullock TL, Breddam K, Remington SJ (February 1996). "Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity". Journal of Molecular Biology. 255 (5): 714–725. doi:10.1006/jmbi.1996.0058. PMID 8636973.
- ^ PDB ENTRY 2xe4 McLuskey K, Paterson NG, Bland ND, Isaacs NW, Mottram JC (December 2010). "Crystal structure of Leishmania major oligopeptidase B gives insight into the enzymatic properties of a trypanosomatid virulence factor". The Journal of Biological Chemistry. 285 (50): 39249–39259. doi:10.1074/jbc.M110.156679. PMC 2998157. PMID 20926390.
- ^ a b c Akhtarshenas, Bahareh; Karami Shabankareh, Hamed; Hajarian, Hadi; Bucak, Mustafa Numan; Abdolmohammadi, Ali Reza; Dashtizad, Mojtaba (2018). "The protease inhibitor antipain has a beneficial synergistic effect with trehalose for ram semen cryopreservation". Reproduction in Domestic Animals. 53 (6): 1359–1366. doi:10.1111/rda.13253. PMID 30011087. S2CID 51629940.
- ^ Nakae K, Kojima F, Sawa R, Kubota Y, Igarashi M, Kinoshita N, et al. (January 2010). "Antipain Y, a new antipain analog that inhibits neurotransmitter release from rat dorsal root ganglion neurons". The Journal of Antibiotics. 63 (1): 41–44. doi:10.1038/ja.2009.109. PMID 19911027.
