User:Jrinehart/Green Sulfur Bacteria: The New Take
| This is not a Wikipedia article: It is an individual user's work-in-progress page, and may be incomplete and/or unreliable. For guidance on developing this draft, see Wikipedia:So you made a userspace draft. Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
Green Sulfur Bacteria
Basic Infromation[edit]
Classification[edit]
Green sulfur bacteria, of the family Chlorobiaceae, are a series of 6 genera: Ancalochloris, Chlorobium, Chloroherpeton, Clathrochloris, Pelodictyon, and Prosthecochloris.
Gram Stain[edit]
All members of the family stain Gram-negative(1).
-
Technical illustration of a Gram Negative Bacteria Cell Wall.
-
Detailed Photo of Green Sulfur Bacteria
Morphology[edit]
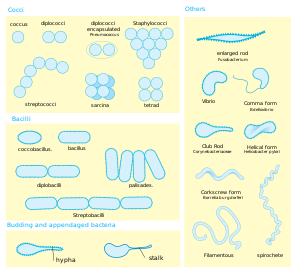
Green sulfur bacteria display a wide range of shapes and sizes and can be coccoid, ovoid, or vibrioid in morphology
Motility[edit]
Species belonging to the chlorobiaceae are usually non-motile and lack flagella; however there is the exception of Chloroherpeton thalassium, which has gliding motility.
Genome[edit]
Very little has been done with DNA sequencing in relation to to green sulfur bacteria; however a lot of focus has been placed on Chlorobium tepidun. Although only ten genomes have been sequenced, these are quite comprehensive of the family's biodiversity. Their 2-3 Mb genomes encode 1750-2800 genes, 1400-1500 of which are common to all strains. The apparent absence of two-component histidine-kinases and response regulators suggest limited phenotypic plasticity. Their small dependence on organic molecule transporters and transcription factors also indicate that these organisms are adapted to a narrow range of energy-limited conditions, an ecology shared with the simpler cyanobacteria, Prochlorococcus and Synechococcus(10)
Preferred Enviroment[edit]
All members of this family occupy a very narrow environmental niche owing to the fact that they are obligate anaerobes as well as obligate photolithotrophs; water cannot serve as an electron donor and instead green sulfur bacteria rely on the reduction of hydrogen sulfide. (1) Metabolic activities and requirements of photosynthetic bacteria determine their etiological niche in the aquatic environment, for example, bacteria found in habitats exposed to low levels of light such as the low hypolimnion of lakes, surfaces of anaerobic sediment and may form planktonic blooms in the anaerobic zones of both freshwater and saline aquatic habitats. They can be found in watery environments, especially where light reaches anoxic water layers, and often can be found in sedimentary layers. Unlike their cousins, the (link)purple sulfur bacteria, they are not easily found in visible accumulations or mats. They are common in sulfur springs, inland lakes, and a large population exists in the Black Sea, one of the largest anoxic water bodies on Earth. (1)
Metabolism[edit]
| Nutritional type | Source of energy | Source of carbon | Examples |
|---|---|---|---|
| Phototrophs | Sunlight | Organic compounds (photoheterotrophs) or carbon fixation (photoautotrophs) | Cyanobacteria, Green sulfur bacteria, Chloroflexi, or Purple bacteria |
Pigments[edit]
Green sulfur bacteria exist in either green or brown colors; green colored species possess bacteriochlorophyll c and d (Bchl c/d) for antenna pigments, while brown colored species contain Bchl e in addition to the carotenoids isorenieratene and b-isorenieratine. Members of the genus Chloroherpeton contain the carotenoid γ-carotene instead. (1) Absorbance spectra of the pigments are as follows: Bchl c (745-755nm); Bchl d (715-745nm); Bchl e (710-725nm). The majority of the antenna pigments in green sulfur bacteria are Bchl c (as much as 97% in Chlorobium tepidum)(2). Higher concentrations of these antenna molecules make members of the family Chlorobiaceae more able to cope with low-light intensities; members of the family are larger than their purple counterparts due to this difference in the number of antenna pigments. It is also noted that the Bchl a : Bchl c ratio increases at lower light intensities and at higher incidence of infrared light (Pringault et al., 1998). Members of the family Chlorobiaceae are able to thrive in environments of as little as 25% of the light intensity needed by the purple sulfur bacteria.
Biosynthesis of antenna pigment Bchl c is not altogether dissimilar from synthesis of Bchl a, the pigment present in the reaction center, and only diverges after formation of the intermediate chlorophyllide a from protoporphyrin IX. The first putative step is elimination of the C-132 methylcarboxyl moiety resulting in a 3-vinyl-8-ethyl-12-methyl Bchlide d, which is likely the first major intermediate of Bchl c and Bchl d. Methylation at C-20 of this or another intermediate form Bchl c (2). Additional methylations at C-82 and C-121 by the genes by the genes bchQ and bchR, respectively, may assist in self-aggregation of Bchl c molecules to control the size of the chlorosome at very low light intensities (2). This aggregation of Bchl c is what allows the chlorosomes of green sulfur bacteria to function at very low light intensities; for example, Chl. phaeobacterioides can be found at the chemocline of the Black Sea at a depth of about 100 meters. The light intensity in this habitat is such that a single chlorophyll molecule will absorb one photon in a period of as much as 6 hours. Thus, there must be very large numbers of Bchl c molecules in the chlorosomes (antennae) of these bacteria; in Chl. tepidum, each chlorosome can contain more than 200x103 Bchl c molecules, and each cell can contain 200-250 chlorosomes.
Photosynthesis[edit]
Photosynthesis is carried out in the cytoplasmic membrane and is capable of cyclic and non-cyclic flow of electrons. The reaction center contains the dimer bateriochlorophyll a called P840. Upon excitation, the reduced P840 becomes a powerful reductant (the reaction potential is approx. -1200mV) and the electrons are transferred by several cofactors, a chlorophyll a isomer (A0, BChl663), amenaquinone-7 (A1, Kjaer et al., 1998), and three iron-sulfur centers (Fx, FA, FB) towards the cytoplasmic side of the membrane. Because of the low redox potential of FA and FB (approx. -540mV), the electrons can be directly transferred to soluble ferredoxin (approx. 410 mV). Ferredoxin is the electron donor in the reductive tricarboxylicacid cycle. Furthermore, NAD+ can be directly reduced by ferredoxin. Therefore a reverse electron flow for NAD+ reduction is not necessary, which partially explains the low energy requirements of green sulfur bacteria. (NOTE all info from article The Family Chlorobiaceae) Cyclic photophosphorylation in the green sulfur bacteria proceeds when the electron reduces menaquinone (MQ) instead of NAD(P)+ and returns to the reaction center via a bc1 complex and cytochrome c555. A proton gradient is created. (Because the standard redox potential of the photosynthetic reaction center in its oxidized state is +240 mV, it cannot oxidize water to O2.) In the noncylcic pathway the electron donor is a reduced inorganic sulfur compound, usually hydrogen sulfide. Elemental sulfur or thiosulfate can also be used. The inorganic sulfur is oxidized by cytochrome c555, which feed electrons into the reaction center (NOTE all previous info from david white book). Because of the considerably shorter span in redox potential, absorption of only about one photon is required per electron transferred to CO2. Fixation of 1 mol C in green sulfur bacteria requires ~4 (3.3–4.5) mol photons (Brune, 1989); thus the quantum yield (mol C assimilated: molquanta absorbed = 0.25) is twice as high as that of purple sulfur bacteria (0.12) or oxygenic phototrophic organisms (maximum, 0.125). (NOTE Family chlorobiaceae article)
Simple model of antenna and reaction-center assembly in green sulfur bacteria (Hauska et al, 2001). Lecture MIP 443 Photsynthesis
References[edit]
1. Overmann, J. 2006. The Family Chlorobiaceae. Prokaryotes, 7:359-378.
2. Frigaard, N., Bryant, D.A. 2004. Seeing green bacteria in a new light: genomics-enabled studies of the photosynthetic apparatus in green sulfur bacteria and filamentous anoxygenic phototrophic bacteria. Archives of Microbiology, 182:265-276.
3. Hauska, G., Schoedl, T., Remigy, H., Tsiotis, G. 2001. The reaction center of green sulfur bacteria. Biochimica et Biophysica Acta, 1507:260-277.
4. Brune, D. C. 1989. Sulfur oxidation by phototrophic bacteria. Biochem. Biophys. Acta 975:189–221.
5. White, David. The physiology and biochemistry of prokaryotes. 3rd. New York: Oxford University Press, USA, 2007. 157-159. Print.
6. Kjaer, B., N. U. Frigaard, F. Yang, B. Zybailov, M. Miller,
7. J. H. Golbeck, and H. V. Scheller. 1998. Menaquinone-7 in the reaction center complex of the green sulfur bacterium Chlorobium vibrioforme functions as the electron acceptor A1. Biochemistry 37:3237–3242.
8.Overmann, J. 2006. The Family Chlorobiaceae. Prokaryotes, 7:359-378.
9. D.A. Bryant & N.-U. Frigaard (November 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends Microbiol. 14 (11): 488. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
10. Beatty JT, Overmann J, Lince MT, Manske AK, Lang AS, Blankenship RE, Van Dover CL, Martinson TA, Plumley FG. (2005). "An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent". Proc. Natl Acad. Sci. USA 102 (26): 9306–10. doi:10.1073/pnas.0503674102. PMC 1166624. PMID 15967984. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1166624.
11. Crowe, Sean; Jones, CarriAyne; Katsev, Sergei; et al., C; O'Neill, AH; Sturm, A; Canfield, DE; Haffner, GD et al. (2008). "Photoferrotrophs thrive in an Archean Ocean analogue". Proceedings of the National Academy of Sciences 105 (41): pp. 15938–43. 2008-10-14. doi:10.1073/pnas.0805313105. ISSN 0148-0227. PMC 2572968. PMID 18838679. http://www.pnas.org/content/105/41/15938.full. Retrieved 2009-06-30
12."Ifree4-research_e." JAMSTEC : Ƨs@lCm¤J@. Web. 01 May 2011. <http://www.jamstec.go.jp/jamstec-e/IFREE/ifree4/research.html>.

