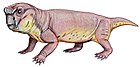
Diictodon
This article needs additional citations for verification. (May 2019) |
| Diictodon | |
|---|---|

| |
| Adult males with distorted skull | |

| |
| Undistorted skull of Diictodon in top view (left) and side view (right) scale bar = 1 cm | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Synapsida |
| Clade: | Therapsida |
| Suborder: | †Anomodontia |
| Clade: | †Dicynodontia |
| Family: | †Pylaecephalidae |
| Genus: | †Diictodon Owen 1876 |
| Species[3][4] | |
| |
| Synonyms | |
| |
Diictodon is an extinct genus of pylaecephalid dicynodont[5] that lived during the Late Permian period, approximately 255 million years ago. Fossils have been found in the Cistecephalus Assemblage Zone of the Madumabisa Mudstone of the Luangwa Basin in Zambia and the Tropidostoma Assemblage Zone of the Teekloof Formation, Tapinocephalus Assemblage Zone of the Abrahamskraal Formation, Dicynodon Assemblage Zone of the Balfour Formation, Cistecephalus Assemblage Zone of the Middleton or Balfour Formation of South Africa and the Guodikeng Formation of China.[6] Roughly half of all Permian vertebrate specimens found in South Africa are those of Diictodon. This small herbivorous animal was one of the most successful synapsids in the Permian period.[7]
Characteristics[edit]
Appearance[edit]

Diictodon had disproportionally large heads that ended in a horny beak. ‘There is a clear distinction between specimens having canine tusks and those lacking them, as tusked specimens are generally larger and more likely to develop a pineal boss. This probably reflects sexual dimorphism, with the tusked sex almost certainly being the male.’ Diictodon had strong arms and legs, as well as 5 sharp claws on each hand, and may have had keen senses of smell and sight. Their gait was similar to the 'high walk' of crocodiles. Their jaws were also simplified, with some of the bones dedicated instead to hearing, considered a key sign of mammalian adaptation. Diictodon also had many adaptations for digging, such as highly developed muscles, a cylindrical body, and wide hands.[8] Researchers Chinsmay and Rubridge analyzed seven other Dicynodonts species discovering the humerual bone microstructure in Diictidon showed no signs of growth marks indicating a variation in its growth strategy that further improved their ability to dig.[9]

Lifestyle[edit]

As a therapsid, Diictodon shared many features with modern-day mammals. Most noticeably, they made burrows into the earth, but most reached up to 0.5 m (1.6 ft) in depth, suggesting that they might have been infrequent diggers and occupied abandoned burrows.[8] Still, many scientists believe that Diictodon lived like the modern gopher. Their burrows could have been used to escape the heat of the desert, which was the dominant environment on the continent of Pangaea in the Late Permian Period. Inside these burrows, nests have been found, where Diictodon skeletons are present. They constituted of quite a gregarious lifestyle with numerous burrows in 500 square meters of space. However, their burrows were unconnected and did not form any large colonies. Many Diictodon nested close to flood plains, and some specimens may have been killed as water flowed into the nests, drowning the animals. Diictidon’s primary utilization of humeral excursion rather than forearm extension aided in employing rotation thrusting when burrowing.[9] Diictodon had no known rival species competing in its niche, so they may have competed primarily with others of their species for the little plant material available.[8] Fossils of infant Diictodon discovered in brood chambers in some burrows suggest there was parental care in the genus, and that males seem to have been involved in raising the infants, based on the fact that some adults in said burrows had tusks.[10]
Diet[edit]
Like all dicynodonts, Diictodon were herbivorous. They used their beaks to break off pieces of the sparse desert shrubs. Like modern desert animals, Diictodon may have had unusually efficient digestive systems, due to the lack of nutrients present in desert plants. As burrowing animals, they may have fed off of water-rich plant tubers.[11]
Sexual dimorphism[edit]
Previously, tusked individuals were considered males, while tuskless individuals were considered females. Differences in pelvic structure may be the other evidence for sexual dimorphism.[8]
Phylogeny[edit]
Diictodon in a cladogram modified from Angielczyk and Rubidge (2010) showing the phylogenetic relationships of Dicynodontia:[12]
| Dicynodontia |
| |||||||||||||||||||||||||||||||||
References[edit]
- ^ M.O. Day, B.S. Rubidge; Biostratigraphy of the Tapinocephalus Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. South African Journal of Geology 2020;; 123 (2): 149–164. doi: https://doi.org/10.25131/sajg.123.0012
- ^ P.A. Viglietti; Biostratigraphy of the Daptocephalus Assemblage Zone (Beaufort Group, Karoo Supergroup), South Africa. South African Journal of Geology 2020;; 123 (2): 191–206. doi: https://doi.org/10.25131/sajg.123.0014
- ^ a b c d e Sullivan, C., & Reisz, R. R. (2005). CRANIAL ANATOMY AND TAXONOMY OF THE LATE PERMIAN DICYNODONT DIICTODON. Annals of Carnegie Museum, 74(1), 45–75.
- ^ Angielczyk, K. D., & Sullivan, C. (2008). Diictodon feliceps(Owen, 1876), a dicynodont (Therapsida, Anomodontia) species with a Pangaean distribution. Journal of Vertebrate Paleontology, 28(3), 788–802.
- ^ Kammerer, C.F.; Angielczyk, K.D. (2009). "A proposed higher taxonomy of anomodont therapsids" (PDF). Zootaxa. 2018: 1–24.
- ^ Diictodon at Fossilworks.org
- ^ Sullivan, C., Reisz, R. R., & Smith, R. M. H. (2003). The Permian mammal-like herbivoreDiictodon, the oldest known example of sexually dimorphic armament. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1511), 173–178. https://doi.org/10.1098/rspb.2002.2189.
- ^ a b c d Ray, S., & Chinsamy, A. (2003). Functional aspects of the postcranial anatomy of the Permian dicynodont Diictodon and their ecological implications. Palaeontology, 46(1), 151–183. https://doi.org/10.1111/1475-4983.00292.
- ^ a b Ray, Sanghamitra; Chinsamy, Anusuya (2004-03-25). "Diictodon feliceps (Therapsida, Dicynodontia): bone histology, growth, and biomechanics". Journal of Vertebrate Paleontology. 24 (1): 180–194. Bibcode:2004JVPal..24..180R. doi:10.1671/1914-14. ISSN 0272-4634. S2CID 86189707.
- ^ Smith, Roger M. H.; Angielczyk, Kenneth D.; Benoit, Julien; Fernandez, Vincent (1 May 2021). "Neonate aggregation in the Permian dicynodont Diictodon (Therapsida, Anomodontia): Evidence for a reproductive function for burrows?". Palaeogeography, Palaeoclimatology, Palaeoecology. 569: 110311. Bibcode:2021PPP...56910311S. doi:10.1016/j.palaeo.2021.110311. S2CID 233585323.
- ^ Cox, C. B. (1998). The jaw function and adaptive radiation of the dicynodont mammal-like reptiles of the Karoo basin of South Africa. Zoological Journal of the Linnean Society, 122(1-2), 349–384. https://doi.org/10.1111/j.1096-3642.1998.tb02534.x
- ^ Kenneth D. Angielczyk; Bruce S. Rubidge (2010). "A new pylaecephalid dicynodont (Therapsida, Anomodontia) from the Tapinocephalus Assemblage Zone, Karoo Basin, Middle Permian of South Africa". Journal of Vertebrate Paleontology. 30 (5): 1396–1409. Bibcode:2010JVPal..30.1396A. doi:10.1080/02724634.2010.501447. S2CID 129846697.
Further reading[edit]
- Ray, Sanghamitra; Chinsamy, Anusuya (January 2003). "Functional aspects of the postcranial anatomy of the Permian dicynodont Diictodon and their ecological implications". Palaeontology. 46 (1): 151–183. Bibcode:2003Palgy..46..151R. doi:10.1111/1475-4983.00292. S2CID 84329913.